视神经损伤
-
Figure 1|OTX2 stimulates axon regeneration from RGCs in vitro.
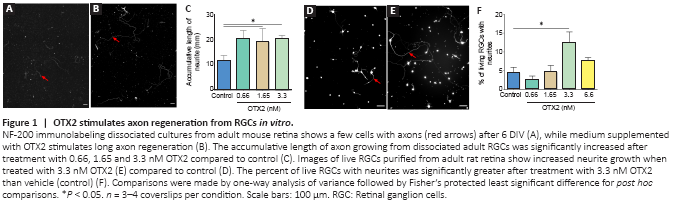
OTX2 stimulated the growth of neurofilament-labeled axons from dissociated adult mouse RGCs in mixed culture (Figure 1A and B). With OTX2, some of the axons were long and tortuous. The measured cumulated axon length per coverslip was significantly increased with OTX2, about doubled at 0.66, 1.65 and 3.3 nM protein compared to vehicle control (Figure 1C). In terms of neuron survival, OTX2 can act directly on neurons in vitro (Torero Ibad et al., 2011; Vargas Abonce et al., 2019). In the mixed culture, OTX2 may act directly on RGCs or indirectly via activity on the other cells present such as bipolar cells, amacrines, Müller glia to stimulate axon regrowth. We used purified cultures of rat adult RGCs to address this question. OTX2 stimulated the growth of neurites from RGCs purified from adult rat retina (Figure 1D and E). We counted the number of live cells and found a significant 5-fold increase at 3.3 nM exogenous OTX2 (not shown). We also counted the number of cells with a neurite with a length at least twice the soma diameter. When normalized to the number of live RGCs, there was a significant 2-fold increase in the number of cells with a neurite (Figure 1F). Since OTX2 increases the survival of RGCs in these conditions (Torero Ibad et al., 2011), these results suggest that OTX2 stimulated neurite outgrowth over and above the increase in survival. These results show that exogenous recombinant OTX2 promotes the growth of axons from dissociated adult RGCs from mouse and rat in vitro, and that RGCs can respond directly to OTX2 for fiber regrowth.
Figure 2|OTX2 stimulates axon growth from explants of adult retina.
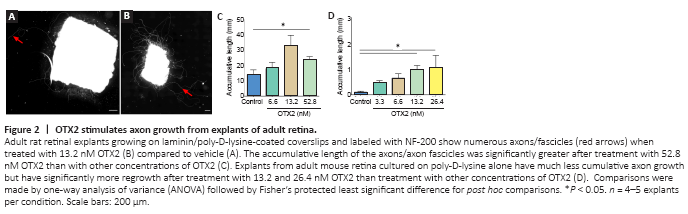
Tissue dissociation shears off axons and dendrites and removes RGCs from their tissue. We then tested whether OTX2 would have an effect on axon growth from explants of adult retina. OTX2 stimulated the growth of numerous neurofilament-labeled axons from adult rat retinal explants. In cultures of vehicle treated explants, axons tended to be isolated and their cumulative length was about 12 mm in 6 days (Figure 2A and C). Explants treated with 13.2 nM OTX2 grew out axons/axon fascicles about three times longer in cumulative length (Figure 2B). This growth is likely to be an underestimate of total growth since this fiber growth included dense bundles formed by multiple axons and thus cumulative individual axon length could be much greater. Substrates of lysine alone compared to lysine/laminin are reported to be less supportive of neurite outgrowth (Yong et al., 1988; Sun et al., 2012). We then wanted to determine if OTX2 could stimulate axon regeneration on a less permissive substrate. The growth of axons from mouse explants cultured on poly-D-lysine without laminin was considerably less but OTX2 still increased cumulative axon growth in a dose-dependent manner and at 24.6 nM this increase was by about 10-fold (Figure 2D). Thus, exogenous OTX2 significantly increases RGC axon regeneration from adult retinal explants even under conditions less favorable to neurite outgrowth.
Figure 3|OTX2 increases RGC survival in vivo after ONC.

We then tested OTX2 in vivo after ONC in adult mice. First, we observed that OTX2 injected soon after ONC significantly increased RGC survival. ONC greatly reduced the density of Brn3a-labeled RGCs compared to an intact retina (Figure 3A–C). Retinas from mice treated with OTX2 clearly had more labeled RGCs. In terms of numbers, whereas adult C57 Bl6 mice typically had about 3300 RGCs/mm2 (Jeon et al., 1998), 14 days after ONC, mice treated with vehicle showed a marked reduction in the number of surviving Brn3a-labeled RGCs with only about one-fifth of that number (Figure 3D). In mice injected with OTX2, the retinas had about a thousand RGCs/mm2, a modest (66%), but significant increase in the number of Brn3A-labeled surviving RGCs compared to vehicle (Figure 3D) and about one-third of the number of RGCs in an intact retina.
Figure 4|OTX2 increases GAP-43-labeled regenerating axons in the optic nerve 14 days after ONC.
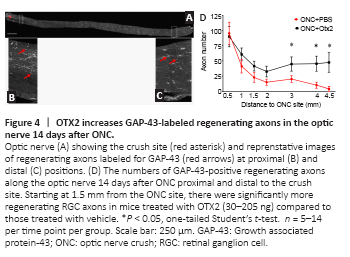
We then evaluated the effect of intraocular injection of exogenous OTX2 on RGC axons after ONC. OTX2 significantly increased and the number of GAP-43-positive fibers/growth cones in the distal optic nerve compared to vehicle injected mice. Longitudinal sections through the optic nerve (Figure 4A) showed bright GAP-43-labled regenerating axons and what are considered to be growth cones were observed along the nerves of OTX2-treated mice (Figure 4B and C). We counted the GAP-43-labled axons along the nerve from the crush site to the chiasm (Figure 4D). Near the crush site nerves from mice in both conditions had similar numbers of labeled fibers (95 for vehicle and 88 for OTX2). In nerves from mice treated with vehicle, the number of GAP-43-labeled profiles diminished with distance with 20 axons at 3 mm from the crush site and 9 axons adjacent to the chiasm (Figure 4D). Starting 1mm from the crush site, mice treated with OTX2 started to show a trend to have more regenerating GAP-43-labeled axons. At more distal regions of the nerve, the number of regenerating axons (about 50) was maintained at least up to the optic chiasm, while in the vehicle-treated mice there was a steady decrease along the nerve. The difference between OTX2-treated mice and the vehicle-treated mice was significant starting 1.5 mm from the nerve crush site and this significant difference continued least up to the optic chiasm 4.5 mm from the crush site (Figure 4D). These results show that OTX2 increased the number of regenerating axons after ONC in vivo over several mm during 2 weeks.
Figure 5|Optic nerve crush reduces visual acuity and OTX2 partially restores visual acuity.
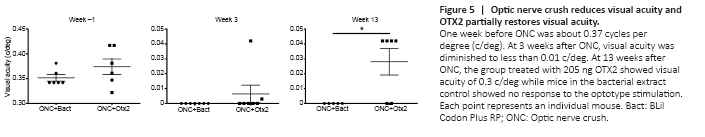
We then examined whether OTX2 treatment could restore visual function. Mice were randomly assigned to two groups to receive bacterial extract or OTX2 injection and then be tested for visual acuity (Figure 5). Optomotry showed that the mean peak acuity was 0.35–0.375 c/deg in each group (Figure 5). At 3 weeks post crush, most mice were blind or showed highly reduced visual acuity (Figure 5; note the change in scale for the Y axis), with 10 mice showing no counterclockwise head movements and two mice responding very poorly. At 13 weeks post crush, the left eye was sutured shut and visual acuity was tested. Four of six mice treated with Otx2 responded to the visual stimuli presented to the right eye, although visual acuity was far inferior to that measured before ONC (note the difference on Y-axis in scale). None of the vehicle treated mice responded to the visual stimuli.
Figure 6|Comparison of effectiveness of RGC regeneration in vivo.
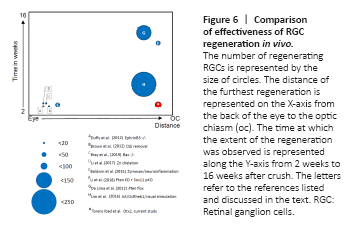
While Otx2 stimulates RGC axon regeneration in vitro and in vivo, is the amount of regeneration meaningful? C57 Blk6 mice have about 50–52,000 RGC axons in the optic nerve (Jeon et al., 1998; Templeton et al., 2014) and these 50–52,000 RGCs and axons underpin all visual behaviors, some of which are complex. Figure 6 compares the number of regenerating axons at their maximal distance after ONC crush obtained with various manipulations in other laboratories. Many strategies resulted in less than 50 axons regenerating to 1–1.5 mm from the ONC site. Duffy et al. (2012) observed 16 axons reaching 1 mm in EphrinB3 knockout mice. Brown et al. (2012) reported similar results in mice in which chondroitin sulfate E was removed. To the best of our knowledge, there are only three previous reports of RGC regeneration reaching the chiasm and only one in the time window that we observed here. de Lima et al. (2012) reported that about 150 axons reached the chiasm 10–12 weeks after ONC after inactivating Pten. Li et al. (2017) used zinc chelation to stimulate regeneration of about 60 axons to the chiasm after 12 weeks. Lim et al. (2016) reported about 180 regenerating axons at the chiasm, 3 weeks after crush, when they enhanced mTOR signaling in addition with high contrast visual stimulation. These approaches are not easily translatable for use in humans.