脑损伤
-
Figure 1|Schematic illustrating the link between mechanically stimulated substance P release, tau hyperphosphorylation and neuroinflammation.
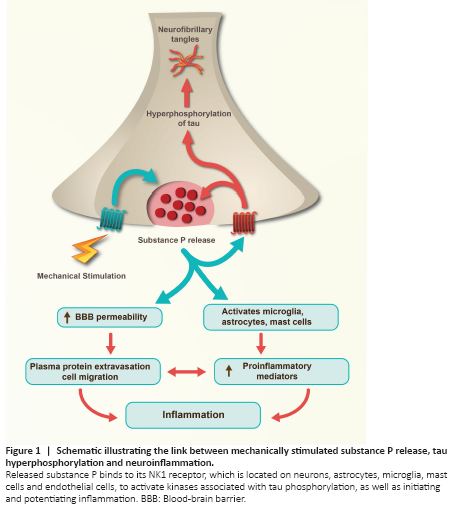
Aside from activating kinases known to be associated with tau phosphorylation, SP is a well-known potent mediator of inflammation (Corrigan et al., 2016). It directly activates microglia, astrocytes, mast cells and leukocytes resulting in the production of various inflammatory mediators including cytokines (interleukin 1, interleukin 6, CCL2), prostaglandins, thromboxane derivatives, kinins, histamine, and nitric oxide (Corrigan et al., 2016). The neuropeptide is also known to increase permeability of the blood brain barrier by upregulating extravasation of vascular proteins, including albumin, which in itself can initiate an inflammatory response. Inflammation has been shown to be a key factor in the development of dementia (Ransohoff, 2016), including CTE. Specifically, cytokines such as CCL2 have been shown to be associated with recruitment of microglia and macrophages and in the development of phosphorylated tau pathology after repeated mild TBI (Cherry et al., 2020), while increased blood-brain barrier permeability has been widely reported following repeated mild TBI and is thought to potentiate ongoing neuroinflammation. Reactive astrogliosis post-TBI is associated with the loss of aquaporin channels, impairing protein clearance via the glymphatic system further contributing to the accumulation of neurotoxic tau species (Graham and Sharp, 2019). Thus, SP release not only leads to perivascular tau hyperphosphorylation, but also initiates and potentiates neuroinflammation (Figure 1).