脑损伤
-
Figure 2|Effects of donor’s gender, tissue source and 10 nM β-estradiol 17-acetate on hBMECs recovery and growth after fresh isolation from neurosurgical specimens.

Upon first plating, GM-derived hBMECs isolated from male patients M1 to M4 spontaneously adhered to the substrate and showed rounded cell clusters associated with the residues of capillary fragments and scattered single cells and debris. These endothelial cells displayed morphological characteristics with typical swirling patterns and whorls and no evident vacuoles, highlighting their overall health. No giant cells or spreading and non-contrasted cells (sign of degeneration) appeared during any growth steps after isolation (see the phase contrast images in Figure 2A). After 1–3 days of culture, cells sprouted from clusters and elongated; after 20 days, confluent colonies of hBMECs isolated from GM of all four male patients were observed. Cultures of WM-derived hBMECs from male patients M2-M adhered and proliferated, but showed lower cell density at confluence compared with GM-derived hBMECs (P < 0.001), even if belonging to the same patient, as reported by the growth curves of Figure 2C.
In contrast, resections of WM from female patients F1, F2 and F3 did not provide adherent or growing cultures, independently of their age, because the addition of β-estradiol 17-acetate to the culture medium of the deriving hBMECs had not yet been considered. In fact, when 10 nM β-estradiol 17 acetate was further added to the growth medium after 2 days in culture, WM-derived hBMECs from patients F4 and F5 interestingly showed strong adhesion and growth capacity significantly exceeding that of GM-derived male hBMECs (P < 0.001) after 10 days in culture (Figure 2C). Therefore, following the addition of β-estradiol 17-acetate, F4- and F5-derived WM-hBMECs displayed a swirling pattern similar to the GM-hBMECs isolated from male patients (see the phase contrast images in Figure 2B), achieving a significant increase in cell number 13 days after isolation (P < 0.001) and confluent colonies after 22 days (Figure 2B). Notably, subcultures of F4- and F5-derived WM-hBMECs treated with the same concentration of DMSO as a vehicle (0.0001%) or with hormone at the concentrations lower than 10 nM failed to adhere or lose their growth behavior compared with 10 nM estrogen-treated endothelial cells. Consequently, these cells were discarded as waste. Therefore, 10 nM has shown the lowest effective concentration of β-estradiol 17-acetate that can stimulate endothelial cell growth in vitro, as also observed in our previous study on dose-dependent release of PGE2 induced by estrogen in human amnion-derived WISH cells (Pavan et al., 2001). Consequently, a 10 nM concentration of β-estradiol 17-acetate was used to maintain the viability of both male and female WM- and GM-derived hBMECs in culture.
The addition of 10 nM β-estradiol 17-acetate to the growth medium of hBMECs after fresh isolation from WM of male patients M4 and M5 induced a significant increase in cell density after 20 days (P < 0.05) compared with the untreated male WM-hBMECs (Figure 2A and C), and clearly never reached the responsiveness to the hormone exhibited by the female WM-hBMECs nor by the male GM-hBMECs after thawing (see below).
Figure 3|Effect of donor’s gender, tissue source and 10 nM β-estradiol 17-acetate on hBMECs recovery and growth after thawing from cryopreservation and splitting.

F4–F5-WM-hBMECs, treated with β-estradiol 17-acetate, were cryopreserved at –80°C for 1 month. Then they were thawed and seeded in growth medium spiked again with 10 nM β-estradiol 17-acetate. β-Estradiol 17-acetate induced a complete recovery of viability of F4–F5-WM-hBMECs and sustained their proliferation until the thirty-third day of culture with routine splitting every 8 days (Figure 3A). Then F4–F5-WM-hBMECs were immediately harvested for subsequent freezing stages. In particular, withdrawal of β-estradiol 17-acetate from the growth medium in separate dishes of F4–F5-WM-hBMECs at the first split 12 days post-thawing decreased their proliferation rate and adhesion to the substrate (Figure 3C). No recovery was induced by a new addition of the hormone after 4 days of estrogenic starvation (Figure 3C inserted panel).
Unlike female WM-derived hBMECs, which adhered and grew to the confluence only in the presence of β-estradiol 17-acetate, cultures of patients M1–M4 GM-derived hBMEC never exposed to the estrogen hormone after fresh isolation grew sufficiently and were thus cryopreserved in freezing medium. However, after thawing and seeding, all the hBMECs deriving from the corresponding patients M1 to M4 showed rapid decreases in adhesion to the substrate and proliferation rate with progressive weakening and loss in culture. Therefore, in order to verify a possible estrogen sensitivity of male hBMECs as far as recovery from freezing is concerned, frozen aliquots of patients M1–M4 GM-derived hBMECs were thawed and seeded in a growth medium added with 10 nM β-estradiol 17-acetate during 32 days of routine subculture. Interestingly, a huge response to estrogens by male GM-derived hBEMCs in terms of viability, cell density at confluence and proliferation rate (Figure 3B) were observed, with a complete recovery occurring even faster than the one observed in hBMECs from female patients, although not statistically different (P > 0.05). Withdrawal of β-estradiol 17-acetate from the growth medium in separate dishes of M1-M4-GM-derived hBMECs at the first split 4 days post-thawing induced a fully reversible slow viability reduction (Figure 3C) when hormone was added back to the culture medium after 8 days of estrogenic starvation.
Figure 4|Cell viability.
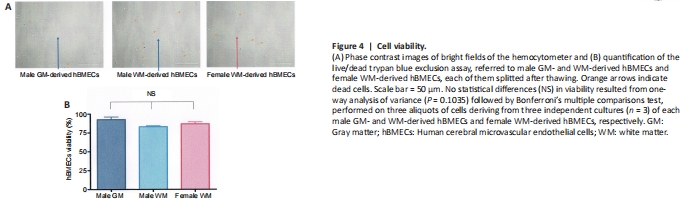
Figure 5|Visualization and quantification of immunofluorescence staining of CD31, CD54 and CD62E markers in representative cultures of male GM- and WM-derived hBMECs and female WM-derived hBMECs.
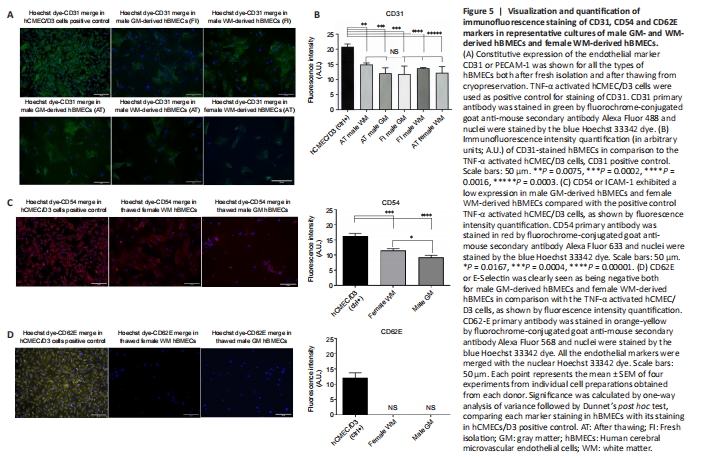
Both female and male patient WM- and GM-derived hBMECs (freshly isolated and after thawing) were stained by immunofluorescence for speci?c surface antigen marker of different endothelial tumor entities. Immunofluorescence staining of all markers was merged with the nuclear Hoechst dye, and compared to their staining in brain endothelial hCMEC/D3 cells activated with 10 ng/mL TNF-α used as positive control (Weksler et al., 2005). All the cultured hBMECs before and after thawing (Figure 5A) showed a detectable expression of PECAM-1 or CD31, which is regarded as the best constitutive endothelial marker. No statistically significant difference (P = 0.9999) in CD31 expression was observed if all types of hBMECs were compared each to other, whereas highly significant differences were observed in comparison with the positive control, as shown in the immunofluorescence quantification (Figure 5B). Both female WM- and male GM-derived hBMECs were stained for cluster of differentiation 54 (CD54) or ICAM-1 (Figure 5C), a 90 kDa member of the immunoglobulin superfamily, which was constitutively expressed in both brain microvascular endothelial cells and umbilical vein endothelial cells (Huber et al., 2006), but it was also normally activated in aged endothelial tissue (Goncharov et al., 2020). TNF -alpha activated hCMEC/D3 cells were a valuable positive control for CD54 marker (Figure 5C), showing a fluorescence intensity 1.5-fold higher than female WM-derived hBMECs (P = 0.0004) and 1.8-fold higher than GM-derived-hBMECs (P = 0.0001), whereas CD54 fluorescence intensity was 1.3-fold higher in female WM-derived hBMECs than GM-derived hBMECs (P = 0.0167). All the cultured hBMECs showed negativity for glycoprotein E-selectin (CD62E), a cell adhesion molecule (CAM), expressed on brain tumor endothelium (Miebach et al., 2013; Jassam et al., 2017), whereas TNF-α activated hCMEC/D3 cells were positive for control of CD62E staining (Figure 5D), confirming the upregulation of CD62E in hCMEC/D3 cells and cancer cells in response to a TNF-α inflammatory stimulus, as reported by other authors (Weksler et al., 2005; Jassam et al., 2016; Dunst et al., 2017).