脑损伤
-
Figure 2|Characterization of hUC-MSCs.
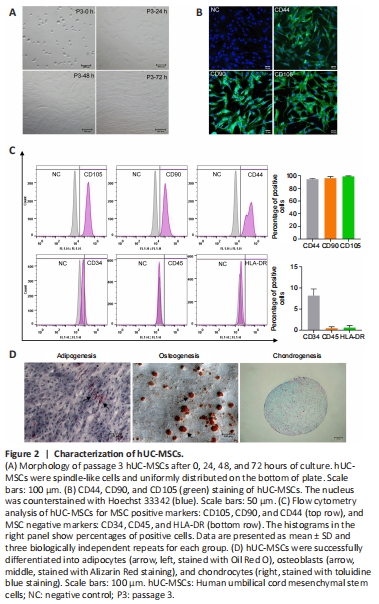
UC tissues from healthy full-term pregnant woman were isolated and cultured, and primary hUC-MSCs were generated as described in Methods. As shown in Figure 2A, hUC-MSCs were arranged in parallel or a spiral shape. Cells were generally passed every 3 days because of their rapid proliferation. Immunofluorescence analysis demonstrated that hUC-MSCs were immunopositive for characteristic markers CD44, CD90, and CD105 (Figure 2B). To further characterize the hUC-MSCs, passage 3 cells were examined human MSC surface markers CD105, CD90, CD44, CD34, CD45 and HLA-DR, as defined by the International Therapy of Cellular Therapy, with flow cytometry. Over 90% of the cells exhibited positive surface expression for CD105, CD90 and CD44; fewer cells were positive for CD34, CD45 and HLA-DR (Figure 2C). These data show that the obtained hUC-MSCs exhibited similar morphology and surface marker expression compared with MSCs. To investigate the differentiation potential of hUC-MSCs, cells were induced into adipocytes, osteoblasts, and chondrocytes. An accumulation of Oil Red O-stained lipid drops was observed in differentiated hUC-MSCs, indicating adipogenesis; osteogenesis was observed with signi?cant calcium deposition determined by alizarin S staining in treated cells, and chondrogenesis was revealed by proteoglycan staining with Alcian blue and was proved by the presence of extracellular matrix formation (Figure 2D). These results showed that the hUC-MSCs had differentiation potential.
Figure 3|NRG1β enhances the differentiation of hUC-MSCs to NSCs.
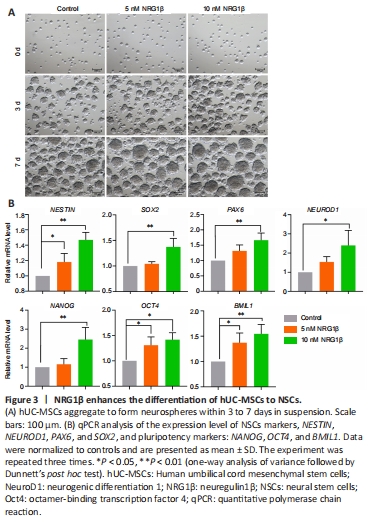
hUC-MSCs were induced into NSCs through the addition of growth factor, and NRG1β was added during induction in the pretreatment group, as described in Methods section. As shown in Figure 3A, neurospheres usually have a round shape, dense core, and clear outline; some cell clusters also show an irregular shape. On the 7th day after induction of hUC-MSCs into NSCs, NSCs were collected and identified by assessing the key markers associated with pluripotency and neural progenitor cells. As shown in Figure 3B, qPCR showed that NSCs expressed the neural progenitor cell markers NESTIN, SOX2, PAX6, and NEUROD1 as well as pluripotency markers NANOG, OCT4, and BMI1.
Figure 4|NRG1β enhances expressions of key markers of NSCs.
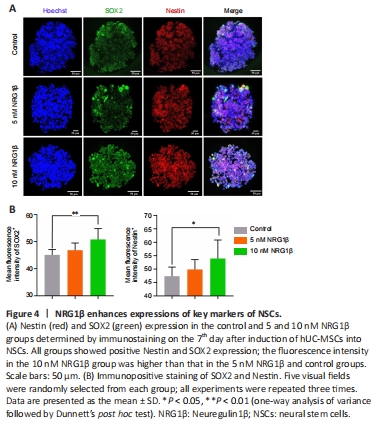
These genes were upregulated in the 5 and 10 nM NRG1β groups compared with the control group, with a larger difference in the 10 nM NRG1β group than that in the 5 nM NRG1β group (P < 0.05 or P < 0.01). Immunofluorescence analysis demonstrated that NSCs were also positive for SOX2 and Nestin, and the expressions of SOX2 and Nestin in the 10 nM NRG1β group were significantly higher than those in the 5 nM NRG1β and control groups (Figure 4A and B; P < 0.01 and P < 0.05). Together, these data suggested that NRG1β enhances the differentiation of hUC-MSCs into NSCs.
Figure 5|NRG1β enhances the proliferation potential of NSCs.

Statistical results showed that the diameter of neurospheres was significantly increased in the 5 and 10 nM NRG1β groups on the 7th day compared with that in the control group (P < 0.05). The percentage of neurospheres over 80 μm in diameter in the 10 nM NRG1β group on the 7th day was higher than those in the control and 5 nM NRG1β groups (Figure 5A). We further performed EdU assays and the results showed that the percentages of EdU-positive cells in the 5 and 10 nM NRG1β groups were significantly higher than that in the control group, with a significantly greater increase in the 10 nM NRG1β group than that in the 5 nM NRG1β group (P < 0.01; Figure 5B). Together, these findings confirmed that NRG1β positively regulates the proliferation of NSCs, and 10 nM NRG1β exhibited superior effects on the generation and proliferation of NSCs compared with 5 nM NRG1β.
Figure 6|Effects of NSCs induced by 10 nM NRG1β on PC12 cell viability after OGD/R.

We next used OGD/R as an in vitro model for I/R injury. Cells were cultured in anaerobic conditions for 2, 4, 6, 8, 10, and 12 hours, followed by reoxygenation under normal conditions for 24, 48, and 72 hours (Figure 6A). Cell viability assays showed that cell viability dramatically declined with increasing OGD time. With reoxygenation treatment, the quality and viability of PC12 cells were also reduced (Figure 6A and B). From these results, we chose OGD for 6 hours and reoxygenation for 48 hours for subsequent experiments.
To examine the effects of NSCs alone or with NRG1β on cell viability during OGD/R, the Transwell system was used to achieve co-culture of PC12 and NSCs. Co-culture condition was given immediately on reoxygenation and continuous for 48 hours; we found that PC12 cells formed an extensive connection in NSCs and NSCs + 10 nM NRG1β groups, but round shape cells and adhesion decreased in the OGD/R group (Figure 6C). The combination treatment led to an improvement in cell viability, and the difference in the viability of the NSCs + 10 nM NRG1β group compared with the OGD/R group was significant (Figure 6D).
Owing to cerebral I/R injury caused ROS generation and as ferroptosis is characterized by the accumulation of lipid ROS, we next examined the level of ROS. The data indicated that compared with ROS level in the OGD/R group, the production of ROS was decreased in the NSCs + 10 nM NRG1β group (P < 0.05; Figure 6E and F). No differences were observed with other groups.
Figure 8|NRG1β relieves PC12 cell damage induced by OGD/R through regulating ferroptosis.
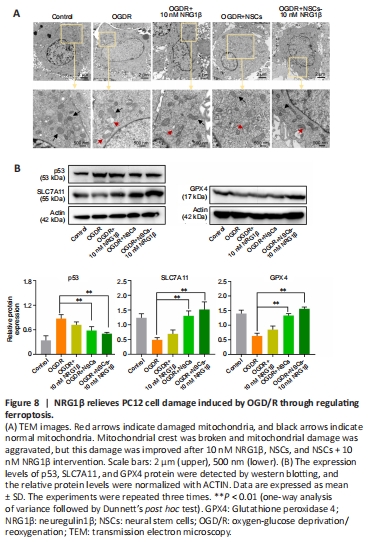
Together, these data showed that pretreated NSCs with 10 nM NRG1β exhibit a strong neuroprotective effect on damaged PC12 cells. Morphologically, ferroptotic cells exhibit typical features of mitochondrial atrophy, diminished or vanished of mitochondrial cristae, and ruptured outer membrane. We also observed smaller mitochondria, increased membrane density and reduction/vanishing of mitochondria in damaged PC12 cells; mitochondrial damage improved in the co-culture groups (Figure 8A).
Studies have shown that p53 suppresses the expression of SLC7A11 (which is the light chain subunit of System Xc–). Reduced SLC7A11 leads to decreased synthesis of GSH and inactivation of GPX4, thus reduces cystine uptake and triggers ferroptosis (Liu et al., 2022). Western blot assay demonstrated that p53 level in the PC12 cells co-cultured with NSCs (OGD/R + NSCs) group and PC12 cells co-cultured with NSCs pretreated with 10 nM NRG1β (OGD/R + NSCs + 10 nM NRG1β) group was lower compared with levels in the OGD/R group (P < 0.01); no differences were observed between the OGD/R and OGD/R + 10 nM NRG1β groups. GPX4 and SLC7A11 levels were lower in the OGD/R group compared with the OGD/R + NSCs and OGD/R + NSCs + 10 nM NRG1β groups (P < 0.01; Figure 8B). These results suggested that the intervention group may play a protective role in reducing the level of ferroptosis in damaged PC12 cells through activating expression of p53, SCL7A11 and GPX4.Figure 9|Effects of NSCs + 10 nM NRG1β on ferroptosis caused by OGD/R in the presence of siRNA-SLC7A11.

To further determine whether the protective role of NSCs pretreated with NRG1β on damaged PC12 cells occurs via the activating pathway of p53/SCL7A11/GPX4, SLC7A11 was knocked down using siRNA-SLC7A11 (Figure 9A and B). Western blotting confirmed a significant decrease in SLC7A11 expression after SLC7A11 knockdown (P < 0.05). We found that the expression levels of p53, SLC7A11 and GPX4 in the NSCs + 10 nM NRG1β group were significantly reversed compared with the OGD/R + siRNA-SLC7A11 group (P < 0.01; Figure 9C). These findings suggested that NSCs + 10 nM NRG1β play an intervention role in reducing levels of ferroptosis in damaged PC12 cells through activating the p53/SCL7A11/GPX4 pathway.