神经退行性病
-
Figure 1|TMEM16F knockdown improves the spatial memory ability of APP/PS1 mice.

We examined TMEM16F expression in the brains of APP/PS1 mice to choose APP/PS1 mice for further behavioral tests and detection of biological indicators. We performed western blot analysis of brain samples from 3-, 6-, 9-, and 12-month-old APP/PS1 mice and age-matched wild-type mice. We found that 6-, 9-, and 12-month-old APP/PS1 mice exhibited high expression of TMEM16F and 9-month-old APP/PS1 mice displayed greatly upregulated TMEM16F levels compared with age-matched wild-type mice (P < 0.01 or P < 0.05; Figure 1A and B). Immunohistochemistry results further displayed the enhanced immunopositivity of TMEM16F in the brains of 9-month-old APP/PS1 mice compared with that of wild-type mice (Figure 1C and D).
Our results indicated that TMEM16F is highly expressed in 9-month-old AD mice. Therefore, we next investigated the roles of TMEM16F in cognitive function by silencing TMEM16F using siRNA-TMEM16F injection into the bilateral hippocampus of 9-month-old APP/PS1 mice and performing Morris water maze tests. Compared with mice in the APP/PS1-NC group, the mice from APP/PS1 siRNA-TMEM16F group exhibited shorter escape latency, more time spent in the target quadrant, and more times of crossing the platform (P < 0.01 or P < 0.05; Figures 1E–H).
Figure 2|TMEM16F knockdown promotes microglial polarization towards M2 phenotype in APP/PS1 mice.

As microglial polarization was closely connected with AD-related neuroinflammation, we next examined the expression of markers related to microglial polarization. Western blot assay revealed that the expression of iNOS, an M1 microglia marker (Yu et al., 2022), was higher in the APP/PS1 group compared with that in the WT group and iNOS was lower in the APP/PS1 siRNA-TMEM16F group, while expression of Arg1, an M2 microglia marker (Yu et al., 2022) was higher compared with those in the APP/PS1-NC group (P < 0.05; Figure 2A and B). Immunofluorescence staining was performed to detect the expression of iNOS in the microglia. The results showed that the number of Iba1+ and iNOS+ cells in the APP/PS1 siRNA-TMEM16F group was higher compared with that in the APP/PS1-NC group (Figure 2C and D).
Figure 3|TMEM16F knockdown inhibits the activation of the microglial NLRP3 inflammasome in APP/PS1 mice.

As activation of microglial NLRP3 inflammasome occurred during the progression of AD, we examined the influence of TMEM16F on the expression of microglial NLRP3 inflammasome. Western blot assay showed that NLRP3 inflammasome was activated in APP/PS1 mice with the upregulation of NLRP3, ASC, and pro-caspase1 compared with the WT group. NLRP3, ASC, and pro-caspase1 expressions were decreased in the APP/PS1 siRNA-TMEM16F group compared with those in the APP/PS1-NC group (P < 0.05; Figure 3A and B). Furthermore, caspase-1 activity was higher in the APP/PS1 group compared with the WT group, and it was greatly inhibited in the APP/PS1 siRNA-TMEM16F group compared with that in the APP/PS1-NC group (P < 0.01 or P < 0.05; Figure 3C and D). Immunofluorescence staining showed that the proportion of Iba1+ and NLRP3+ cells in the hippocampus and cortex decreased (Figure 3E and F). These outcomes showed that TMEM16F knockdown prevents NLRP3 inflammasome activation.
Figure 4|TMEM16F knockdown decreases the secretion of pro-inflammatory cytokines and repairs morphological injury of microglia.
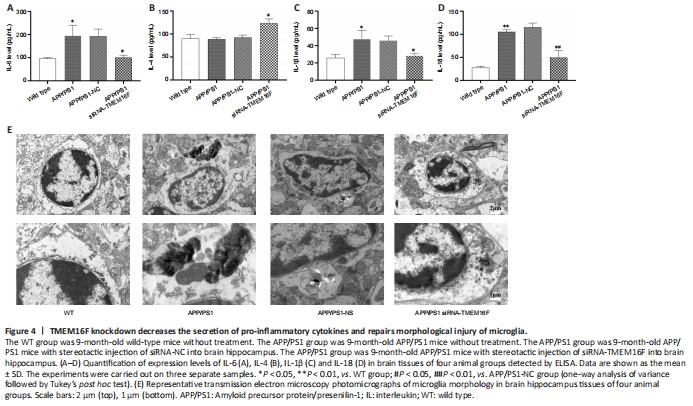
Because the secretory patterns of inflammatory cytokines will change according to microglial phenotype, we further examined the secretion of inflammatory cytokines. ELISA results showed that secretion of pro-inflammatory IL-6, IL-1β, and IL-18 from brain tissue in the APP/PS1 was increased compared with the WT group, and the secretory level of IL-6, IL-1β, and IL-18 in the APP/PS1 siRNA-TMEM16F group was decreased compared with the APP/PS1-NC group, while the level of anti-inflammatory IL-4 increased (P < 0.01 or P < 0.05; Figure 4A–D). TEM was performed to observe the ultrastructure of hippocampal tissue. TEM revealed that microglia from the APP/PS1 and APP/PS1-NC groups demonstrated ruptured cell membranes with irregular shapes and exhibited concentrated chromatin with the secretion of cytoplasmic content, which was partially reversed after TMEM16F knockdown by the injection of siRNA-TMEM16F (Figure 4E).
Figure 5|TMEM16F knockdown attenuates neuronal apoptosis and pathological injuries in APP/PS1 mice.

Western blot assays were performed to evaluate the level of apoptosis-related proteins in the brain tissue of APP/PS1 mice. Bax, bcl-2, and cleaved-caspase-3 expressions were greatly in tissues with downregulated expression of TMEM16F (P < 0.01 or P < 0.05; Figure 5A–D). Immunohistochemistry revealed decreased expression of caspase-3 in cortex and hippocampus regions of brains in APP/PS1 siRNA-TMEM16F mice. The deposition of Aβ plaque can reflect the degree of pathological injuries. Immunohistochemistry results revealed that the deposition of Aβ plaque was greatly increased in the APP/PS1 and APP/PS1-NC groups, and these levels were decreased by siRNA-TMEM16F injection (P < 0.01 or P < 0.05; Figure 5E and F). Finally, hematoxylin and eosin staining was performed to determine pathological changes of neurons in brain tissue samples,. The results showed sparse and disorganized neurons with pyknotic blue nuclei in the 9-month-old APP/PS1 and APP/PS1-NC groups. In comparison, the morphology of neurons in the APP/PS1 siRNA-TMEM16F group was improved (Figure 5G).
点击此处查看全文