脑损伤
-
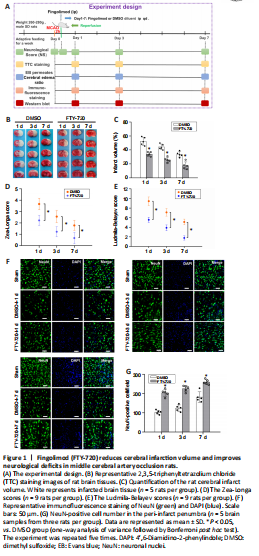
Figure 1|Fingolimod (FTY-720) reduces cerebral infarction volume and improves neurological deficits in middle cerebral artery occlusion rats.
The extracted cerebral microvessels were added to 60 μL RIPA lysis buffer (brain tissue added at 1:4), homogenized, lysed for 30 minutes, centrifuged at 16,000 × g for 20 minutes, and the precipitate discarded. The protein concentration was then evaluated using a BCA protein assay (Cat# 23227, Thermo Fisher Scientific). After boiling using a constant temperature bath (Monad Biotech Co., Ltd., Wuhan, Hubei Province, China), 10–12% sodium dodecyl-sulfate polyacrylamide gel electrophoresis (SDS-PAGE; Solarbio Science & Technology Co., Ltd.) was used to separate the total proteins (30 μg), which were then transferred onto polyvinylidene difluoride (PVDF) membranes (Millipore, Bedford, MA, USA). The membranes were blocked for 1 hour with 5% skimmed milk (Solarbio Science & Technology Co., Ltd.) in TBST (tris-buffered saline and Polysorbate 20, also known as Tween 20) at room temperature, followed by overnight incubation at 4°C with the following primary antibodies: rabbit anti-occludin antibody (tight junction protein, 1:1000; Cat# 40-4700, Thermo Fisher Scientific), rabbit anti-claudin-5 polyclonal antibody (tight junction protein, 1:1000; Cat# PA5-99415, Thermo Fisher Scientific), rabbit anti-S1PR1 polyclonal antibody (endothelial cell receptor; 1:1000; Cat# ab11424, Abcam), rabbit anti-IL-17A polyclonal antibody (an inflammatory cytokine; 1:1000; Cat# PA5-106856, Thermo Fisher Scientific), mouse anti-β-actin polyclonal antibody (1:3000; Cat# 3700, CST). After incubation with the primary antibody overnight, the membranes were further incubated with goat anti-rabbit IgG (1:15,000; Cat# SE134, Solarbio Science & Technology Co., Ltd.)/goat anti-mouse IgG (1:15,000; Cat# SE131, Solarbio Science & Technology Co., Ltd.) secondary antibodies at room temperature for 2 hours. The membranes were visualized with an enhanced chemiluminescence system (Beyotime, Shanghai, China) to display the protein bands and a ChemiDoc-It TS2 Imager (UVP, LLC, Upland, CA, USA) was used to capture the images. ImageJ was used to perform densitometric analysis. The relative protein expression was normalized to β-actin. The above experimental design is shown in Figure 1A.
To determine whether FTY-720 exerted a protective mechanism on cerebral ischemia/reperfusion injury in rats, TTC staining was performed at 2 hours after ischemia and 1, 3, and 7 days after reperfusion, and the volumes of cerebral infarction before and after FTY-720 treatment were compared. At 1, 3, and 7 days after reperfusion, the infarct volume in the ischemic hemisphere of the FTY-720 group was significantly lower than that in the DMSO group (P < 0.05; Figure 1B and C). Evaluation of the neurological deficits using the Zea-Longa (Figure 1D) and Ludmila-Belayev scores (Figure 1E) revealed that the neurological function of the rats was significantly better in the FTY-720 group than that in the DMSO group (P < 0.05).
To further determine whether FTY720 could reduce the severity of neuron injury, neurons were identified using NeuN staining for neurons (Figure 1F). The results showed that the neurons in the peri-infarct penumbra were higher in the FTY-720-treated group than in the DMSO group (P < 0.05; Figure 1G).
Figure 2|Fingolimod (FTY-720) reduces ischemia-induced blood-brain barrier disruption and edema.
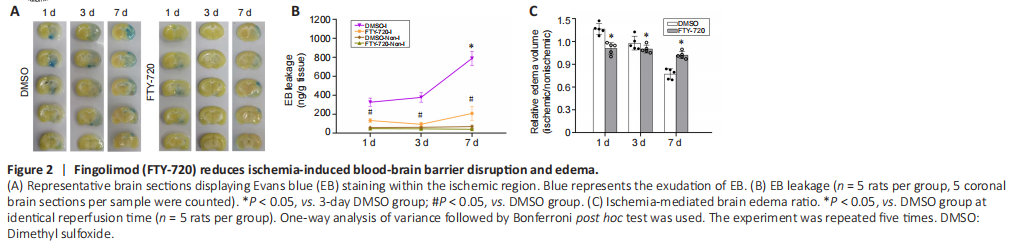
Next, we employed two different methods to evaluate the effect of FTY-720 on ischemia-induced BBB disruption: EB extravasation and relative cerebral edema volume. Figures 2A and B show EB extravasation at 1, 3, and 7 days after reperfusion. As expected, the EB contents of the nonischemic hemispheric tissues were low at all timepoints. Cerebral ischemia caused a remarkable increase in the EB contents of the ischemic hemispheric tissues in the DMSO group. This considerable EB leakage was accompanied by a longer reperfusion duration. At all three reperfusion timepoints, the FTY-720 treatment reduced the EB extravasation in comparison with the DMSO group (P < 0.05).
Cerebral edema is another indicator used to assess BBB injury caused by cerebral ischemia. Figure 2C illustrates results using the ratio of the enlargement of the ischemic hemisphere to reflect the severity of brain edema, and it can be observed that brain edema was largest at 1 day after reperfusion, and that the edema of the ischemic hemisphere had disappeared on day 7. After FTY-720 treatment, the enlargement rate of the ischemic hemisphere was significantly lower than that in the DMSO group, and the atrophic brain tissue was also effectively improved on day 7 (P < 0.05).
Figure 3|Fingolimod (FTY-720) reverses tight junction protein losses in ischemic microvessels of ischemic stroke rats.
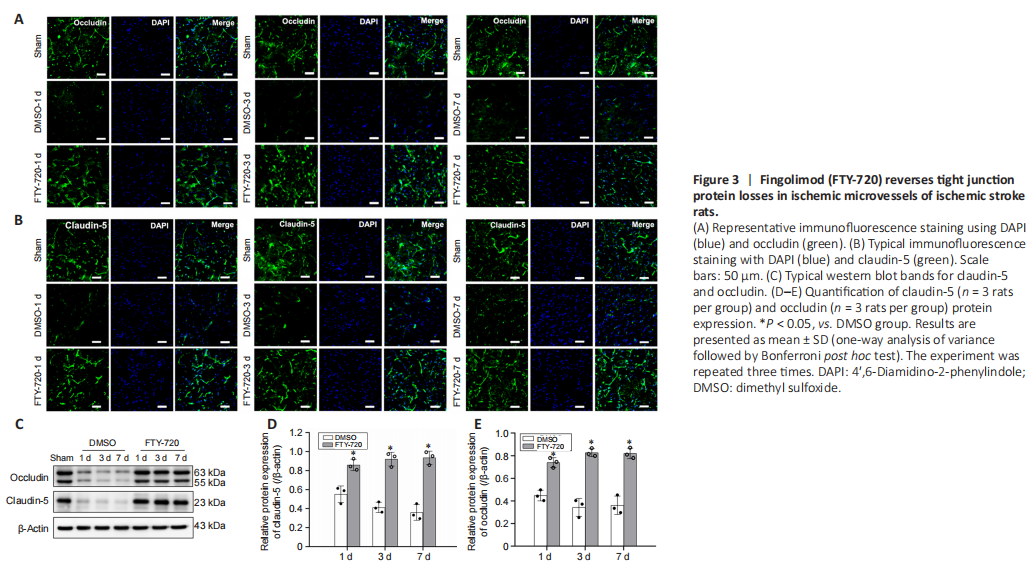
Next, to explore whether FTY-720 modulated TJP levels, occludin and claudin-5 immunoreactivities were estimated using immunohistochemistry methods. The results showed that occludin and claudin-5 immunoreactivities were weakened in ischemic rats (DMSO group), while immunoreactivities were obviously observed after FTY-720 treatment (Figure 3A and B). We also used western blotting to evaluate the occludin and claudin-5 protein levels of the isolated microvessels. The results indicated declines in occludin and claudin-5 protein expression post-ischemia, whereas FTY-720 increased occludin and claudin-5 protein expression in the microvessels of the ischemic stroke rats (Figures 3C–E).
Figure 4|Fingolimod (FTY-720) alleviates ischemia/reperfusion-induced cerebral microvascular damage by acting on microvascular endothelial S1PR1.
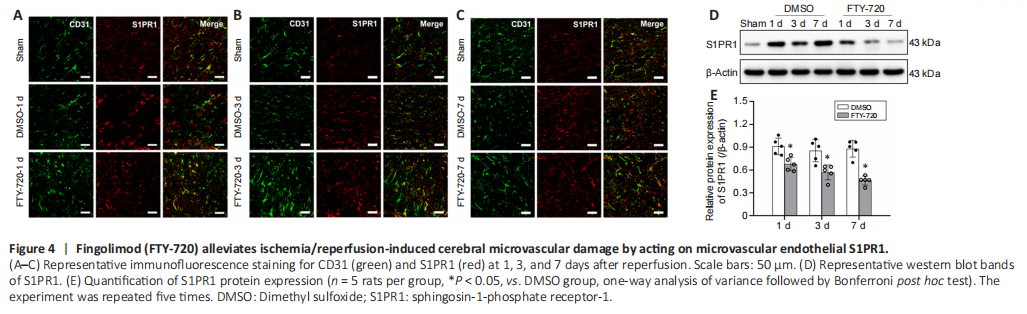
To gain a deeper insight into the protective impact of FTY-720 on cerebral microvessels, we observed CD31 (microvascular endothelial cells marker) and S1PR1 co-staining using immunohistochemistry methods. We observed that the connectivity of the cerebral microvessels was markedly lost after ischemia and that colocalization with S1PR1 was reduced (Figure 4A–C). After FTY-720 treatment, the connectivity of the cerebral microvessels was intact and there were several small branches. These results revealed that FTY-720 exhibited a protective role on cerebral microvessels by acting on microvascular endothelial S1PR1.
We additionally used western blotting to assess the protein levels of S1PR1 in isolated microvessels, the results of which also indicated that FTY-720 treatment significantly reduced the expression of S1PR1 on cerebral microvessels compared with the DMSO group (P < 0.05; Figure 4D and E). The morphology and continuity of the cerebral microvessels in the ischemic hemisphere remained intact after FTY-720 treatment (Figure 4A–C), which may have maintained vascular continuity and promoted cerebral microvascular angiogenesis by acting with S1PR1 on the microvascular endothelial cells.
Figure 5|Fingolimod (FTY-720) attenuates ischemia/reperfusion-induced glial cell activation and IL-17A expression.

To further comprehend the NVU status following ischemia, astrocytes and microglia activation were assessed by immunohistochemistry. First, we co-stained GFAP and IL-17A and observed ischemia-induced astrocyte activation, morphological changes, and apparent colocalization with IL-17A (Figure 5A). Moreover, FTY-720 treatment improved the activation of astrocytes and decreased colocalization with IL-17A. Next, we performed fluorescent staining of Iba-1 and IL-17A and recorded similar results to those mentioned above (Figure 5B).
To further confirm that FTY-720 inhibited the expression of inflammatory factors, we also used a western blot to assess the protein levels of IL-17A in brain tissue. This showed that FTY-720 effectively diminished the expression of IL-17A (Figure 5C and D).
Figure 6|Fingolimod (FTY-720) decreases neuronal apoptosis in the peri-infarct penumbra of rats after ischemia/reperfusion.
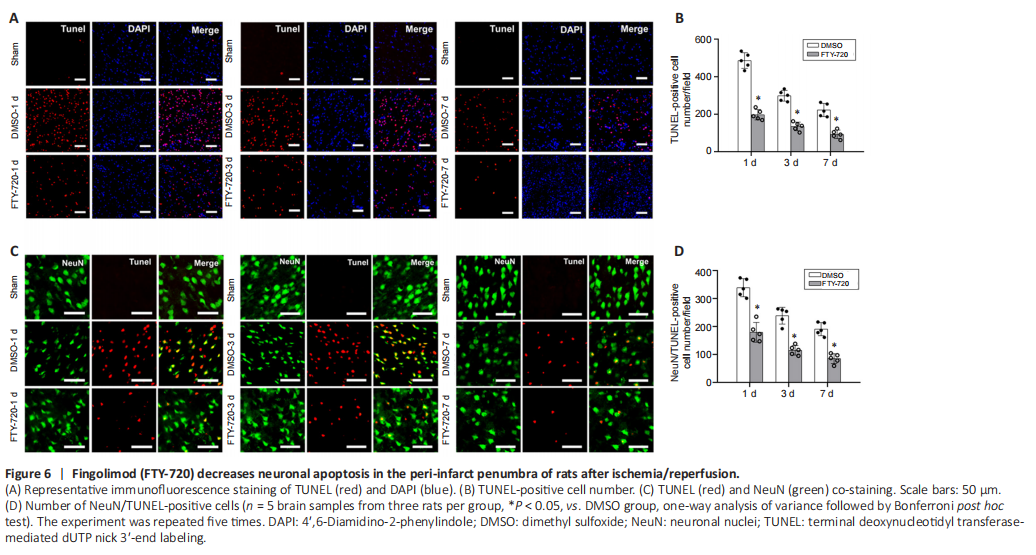
To elucidate the protective mechanism of FTY-720 on ischemia/reperfusion-induced NVU damage, we used TUNEL staining to explore how FTY-720 affected neuronal apoptosis. We discovered that compared with the DMSO group, TUNEL-positive cells (which are used to quantify apoptotic cells) and the amounts of apoptotic cells decreased significantly after FTY-720 treatment (P < 0.05; Figure 6A and B). TUNEL staining was negative in the brain sections of the sham group, whereas the amounts of TUNEL-positive neurons were greater in the DMSO group during ischemia/reperfusion than in the FTY-720 group, implying that FTY-720 treatment could effectively decrease the number of TUNEL-positive neurons compared with the DMSO group (P < 0.05; Figure 6C and D).