神经退行性病
-
Figure 3|Glial cell line-derived neurotrophic factor (GDNF) reverses cognitive deficits induced by MPTP, and a decrease in GDNF level in the prefrontal cortex (PFC) directly leads to cognitive impairment.

To examine the correlation between the PFC and blood levels of GDNF in PD mice, we compared GDNF level changes after demonstrating that MPTP successfully induced the loss of DANs in the substantia nigra. Mice showed a deficit in working memory/avoidance memory performance as well as a GDNF reduction in both the PFC and serum. There was a significant positive correlation between decreased GDNF and cognitive dysfunction in mice (Additional Figure 2). To gain further insight into the effects of endogenous GDNF loss in the PFC on cognition, we used a unilateral stereotaxic injection to deliver AAV-mediated GDNF knockdown into the PFC of C57BL/6J mice (Figure 3A–P) and assess whether the reduction in the GDNF level impairs cognition, thereby mimicking the initial onset of PD. We examined the primordial extent of viral transfection in the PFC (Figure 3A) and found that AAV-GDNF-RNAi (AAV-RNAi) specifically reduced GDNF levels in the PFC (Figure 3D, E, and K). Short-term spatial working memory was altered in AAV-RNAi-treated mice, reflected by their similar spontaneous alternation behavior during the Y-maze task to control mice (Figure 3L and M). In the passive avoidance reproduction memory test, AAV-RNAi-treated mice tended not to spend a prolonged period in the light compartment (Figure 3M). These clinical results suggested that the serum GDNF level is positively associated with cognitive performance (Figure 3N–P).
Figure 5|Effect of glial cell line-derived neurotrophic factor (GDNF) on dopamine transporter (DAT), postsynaptic density protein 95 (PSD95) expression, and dopaminergic synaptic morphological structure.
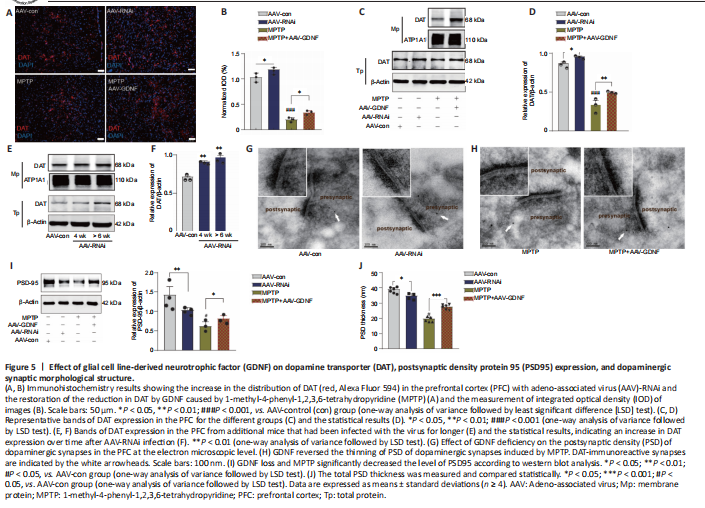
Moreover, this group exhibited a prolonged decay of signals to baseline (> 4 seconds), as reflected by the size of the slope (Figure 4F), whereas the signals of the control group returned to baseline rapidly in approximately 2 seconds. In contrast, the DA1h signal of the AAV-RNAi group peaked at the end of the 1.7-second stimulation and sharply decreased to the pre-stimulation level in 3 seconds (Figure 4G). Furthermore, the slope of the signal returning to the pre-stimulation level in the AAV-RNAi group was higher than that in the AAV-con group (Figure 4G). Overall, the fluorescence signals in the AAV-RNAi group were lower than those in the control and AAV-GDNF groups (Figure 4G). However, because of the regulation effect of GDNF on DAT, the levels of DA in the synaptic cleft were not consistent, which contributed to the differences in the DA1h signal value and the signal decay time. We found a significant increase in DAT levels in the AAV-RNAi group, and the enrichment of DAT in the membrane was most evident in this group (Figure 5A–F). Notably, when we assessed the structure of dopaminergic synapses in the PFC in more detail via immune electron microscopy, there was insufficient evidence to indicate significant DAT level changes in the dopaminergic presynaptic components. However, the thickness and volume of the postsynaptic dense area showed slight variations, which appeared as thinning in the AAV-RNAi group (Figure 5G). Furthermore, the synaptic marker PSD95 was decreased, which corresponded to the elevated DAT in the AAV-RNAi group (Figure 5C and I). These results indicated that GDNF deficit-induced DAT enrichment involves reductions in effective DA transmission and dopaminergic synapse modification.
Figure 6|Glial cell line-derived neurotrophic factor (GDNF) altered long-term potentiation (LTP), mediated by dopamine (DA) level, dendritic spine density, and morphology of the prefrontal cortex (PFC).
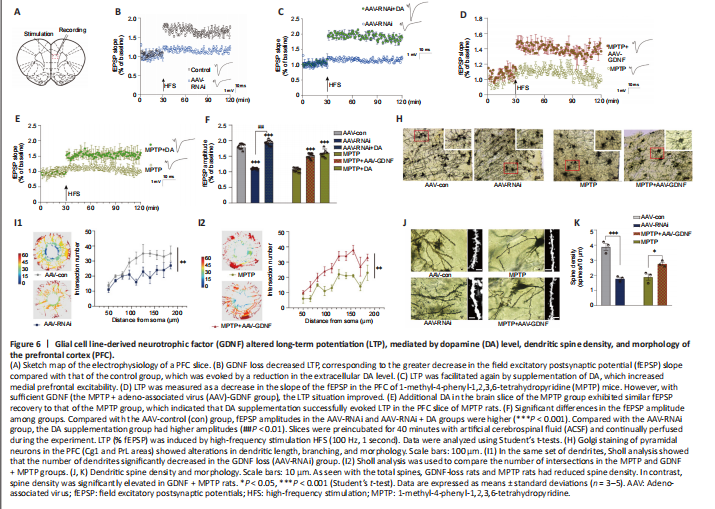
Both GDNF and DA signaling modulate synaptic plasticity (Chen et al., 2008; Kopra et al., 2017). However, the plasticity of excitatory glutamatergic neurons is rarely reported, especially in the PFC. The above observations prompted us to test whether a decrease in GDNF results in a reduction in LTP (Figure 6A–F). We found that the AAV-RNAi group had a greater deficit in LTP than the AAV-con group (Figure 6B). To test whether an increase in DA concentration ameliorates this LTP deficit in AAV-RNAi mice, we added DA into the ACSF and acquired electrophysiological recordings. Appropriate DA supplementation ultimately rescued LTP in the GDNF-deficit mice (Figure 6C). As shown in Additional Figure 3, the fEPSP amplitude was significantly increased by low-dose DA (< 250 nM) and was significantly decreased once high-dose DA was administered (> 250 nM).
Differences in the change in expression of the synaptic marker PSD95 (Figure 5I and J), according to the above results and LTP (Figure 6A–F), suggested that the change is associated with spine density (Figure 6H–K). The dendritic spines were abundant and uniformly distributed (Figure 6J), the boundary of spines was clear, and the morphology was predominantly mushroom-shaped, long, and thin. The AAV-RNAi-treated mice showed a marked reduction in spine density in the PFC (Figure 6J and K). In addition, the dendrites became swollen, and the boundary of the spines became blurred. Notably, we observed that the structure of the dendritic tree of the pyramidal neurons tended to exhibit fewer intersections, and only the apical dendrites with relatively distinct features were retained in AAV-RNAi mice, as observed in the Sholl analysis (Bird and Cuntz, 2019; Figure 6H and I).
Figure 7|Glial cell line-derived neurotrophic factor (GDNF) decreases the loss of dopaminergic axons from the ventral tegmental area (VTA) in the prefrontal cortex (PFC).
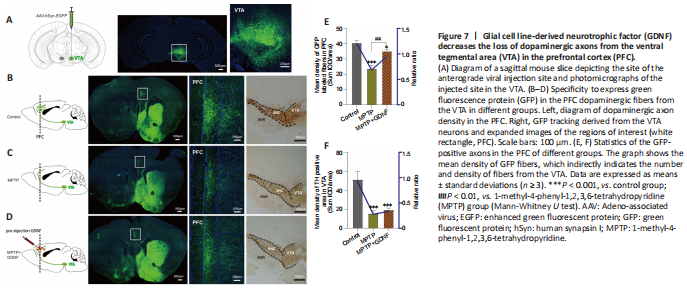
Furthermore, we used DA dynamics recordings to detect electrically-evoked DA release in the PFC to examine how MPTP affects DA output in the PFC. We found a significant decrease in the peak amplitude of evoked extracellular DA released in MPTP mice (Additional Figure 4A–C). Significantly more DA was released following the same stimulation when there was sufficient GDNF, and DA had a longer-lasting effect (Additional Figure 4D). Furthermore, we administered an anterograde AAV-syn-enhanced green fluorescent protein (EGFP) injection into the VTA and observed a decrease in dopaminergic fibers in the PFC of PD mice (Figure 7A–C). However, when the GDNF factors were injected early, the PFC received more EGFP-positive fibers (Figure 7D–F); thus, the diminished dopaminergic innervation was reversed. With the loss of dopaminergic fibers, western blotting (Figure 5A–D) and the HPLC assay (Figure 4C and D) showed that the DAT and DA levels were inevitably reduced, which also explains the reduction in DA delivery. It is well-established that the loss of dopaminergic projections profoundly affects cognitive flexibility, which is consistent with our behavioral results.