NRR:上海交大医学院陈浩团队揭示microRNA181b在缺血性脑卒中血管新生中的作用及调控机制
撰文:薛丽霞、陈浩
缺血性脑卒中因发病率、致残率以及死亡率高,而严重危害人类健康。阐明脑缺血后血管新生分子调控机制,寻找有效措施促进血管新生,有望改变目前缺血性脑卒中治疗的困境。非编码微小RNA作为缺血性脑卒中的潜在治疗靶点受到广泛的关注。研究发现,miR-181b可通过调控缺氧诱导因子1α表达,促进血管生成[1, 2],抑制创伤性脑损伤后的神经炎症反应[3]。既往研究表明,PTEN抑制可通过促进Akt磷酸化和缺氧诱导因子1α表达,促进血管新生[4]。由于PTEN作为miR-181b的潜在靶基因,miR-181b可通过抑制其表达而促进Akt的磷酸化[5]。然而,目前尚未阐明miR-181b是否也能通过PTEN/Akt信号通路参与调控缺血性脑卒中后血管新生,从而促进神经功能恢复。
近日,中国上海交通大学医学院陈浩团队在《中国神经再生研究(英文版)》(Neural Regeneration Research)上发表了题为“miR-181b promotes angiogenesis and neurological function recovery after ischemic stroke”的研究论文。研究发现,在体外氧糖剥夺模型中,miR-181b过表达能够提高受损脑微血管内皮细胞的活性,增强其血管形成能力;在局灶性脑缺血大鼠模型中,miR-181b过表达也能缩小脑梗死体积,促进缺血半暗带中血管新生,从而改善神经功能。进一步研究证实,miR-181b可通过直接与PTEN的3'非翻译区结合,诱导PTEN降解,激活Akt通路,上调血管内皮生长因子表达以及下调内皮抑素表达,从而促进血管生成的。因此外源性miR-181b有望成为脑卒中血管新生治疗的潜在策略。
陈浩等首先建立了动物局灶性脑缺血模型,明确外源性miR-181b对缺血性脑卒中的神经保护作用。可见外源性miR-181b能减少局灶性脑缺血大鼠脑梗死体积,改善其神经功能(图1),并通过抑制PTEN激活Akt信号通路,促使血管内皮生长因子表达上调,同时抑制内皮抑素表达,从而促进缺血半暗带中血管新生(图2)。在体外脑微血管内皮细胞氧糖剥夺模型中,miR-181b可恢复氧糖剥夺诱导的脑微血管内皮细胞的活性,减少细胞凋亡,增强其体外血管生成能力(图3)。为进一步探究miR-181b调控PTEN参与缺血后脑卒中血管新生的分子机制,实验通过双荧光素酶报告基因实验检测,发现miR-181b可调控带有PTEN 3'非翻译区荧光素酶的表达;而在结合位点突变后,这种调控关系消失,这证实了miR-181b是通过该结合位点而实现调控PTEN表达的(图4)。综上,外源性miR-181b对缺血性脑卒中有神经保护作用,且其作用与PTEN/Akt信号通路激活,促进血管新生有关。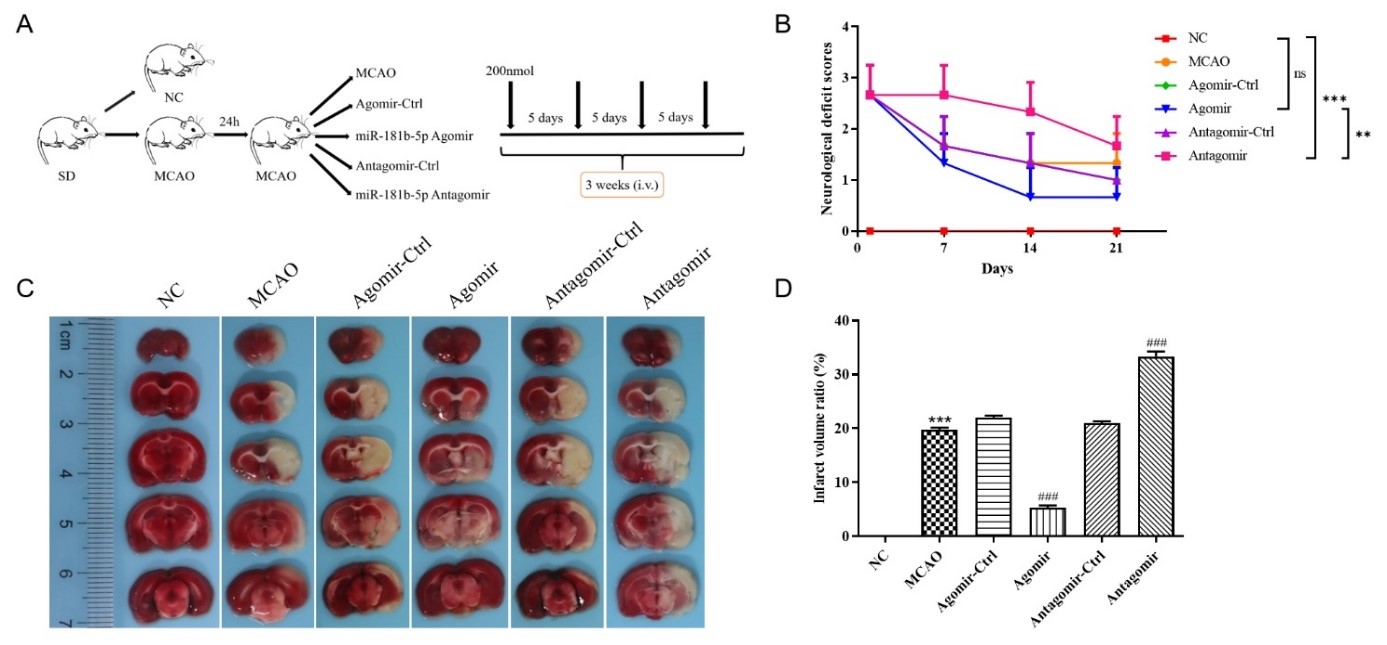
图1. 外源性miR-181b可缩小局灶性脑缺血大鼠脑梗死体积,促进神经功能恢复(图源:Xue et al., Neural Regen Res, 2023)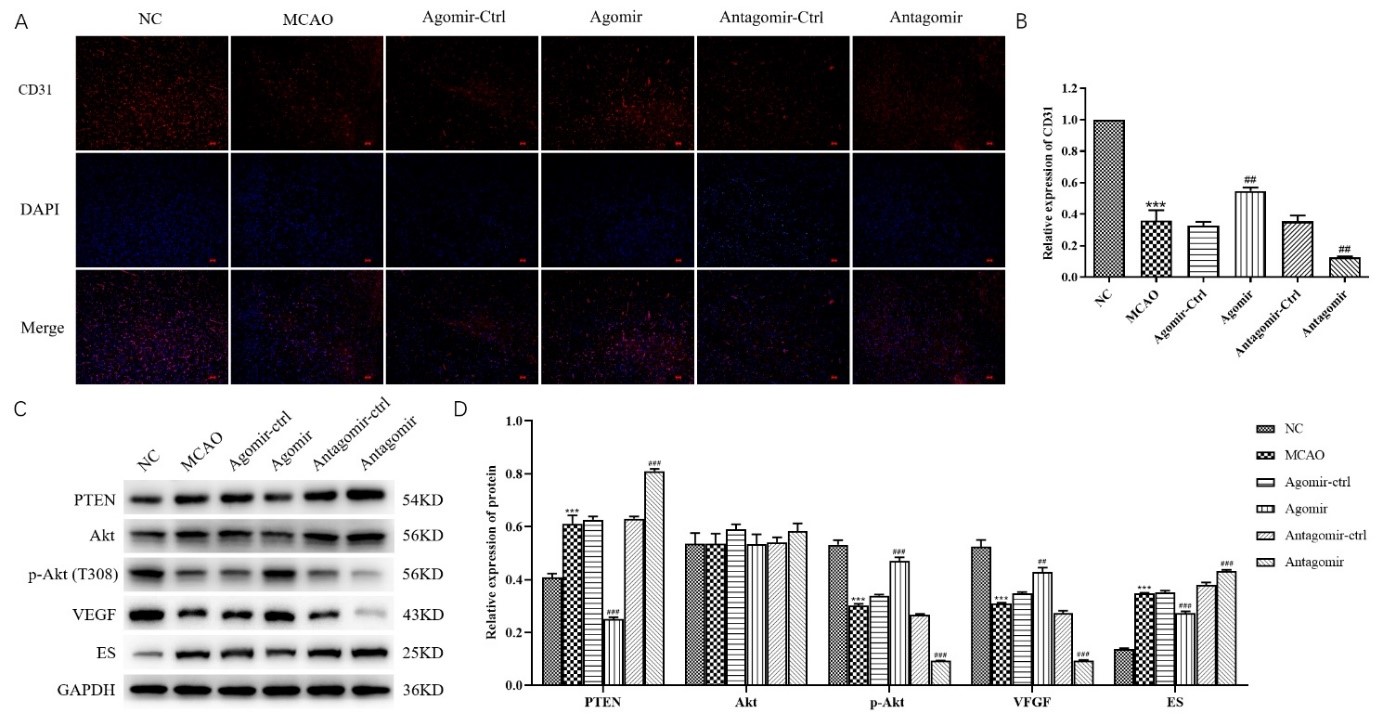
图2. miR-181b可通过激活PTEN/Akt信号通路促进局灶性脑缺血后缺血半暗带血管新生(图源:Xue et al., Neural Regen Res, 2023)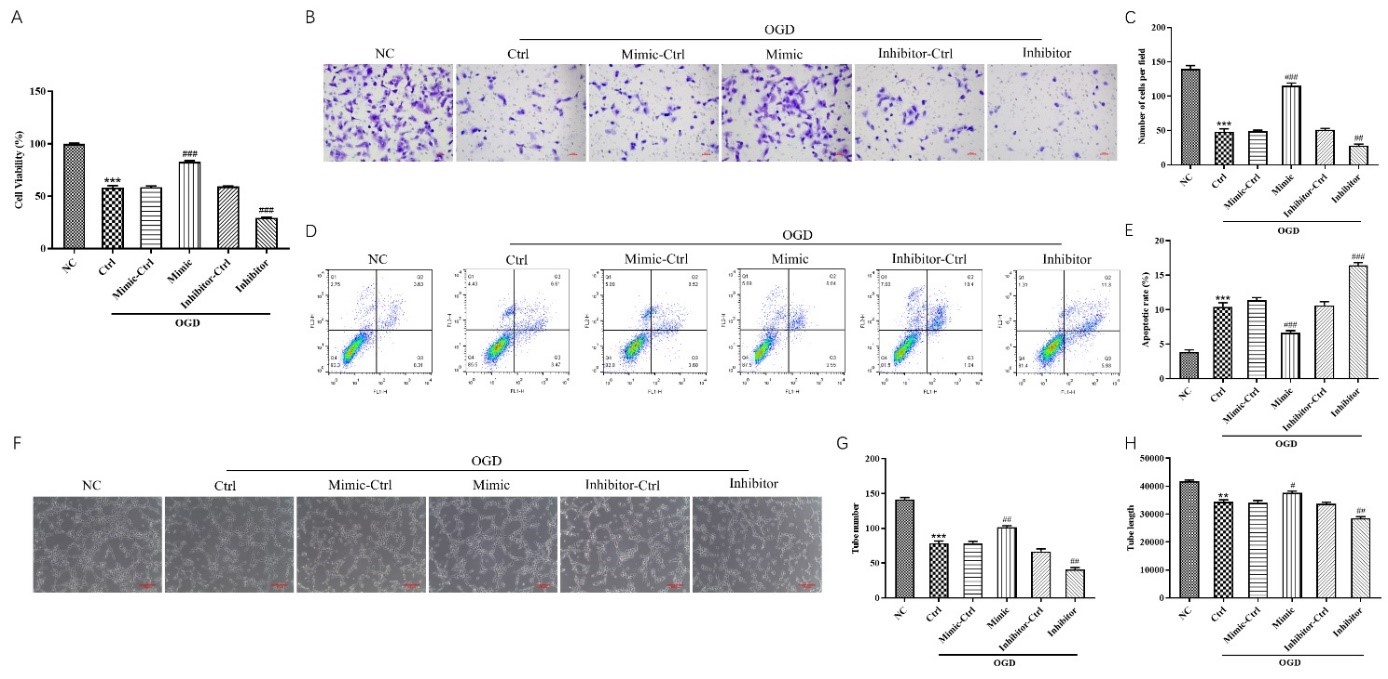
图3. miR-181b增强氧糖剥夺诱导的脑微血管内皮细胞的活性、迁移和血管生成能力(图源:Xue et al., Neural Regen Res, 2023)

图4. 双荧光素酶报告基因实验验证miR-181b与PTEN的结合位点(图源:Xue, et al. Neural Regen Res, 2023)
综上所述,miR-181b可通过与PTEN结合来激活Akt信号通路,从而调控血管内皮生长因子和内皮抑素表达,进而促进缺血性脑卒中血管新生,表明miR-181b/PTEN信号轴可能是缺血性脑卒中新治疗靶点,而外源性miR-181b有望成为脑卒中血管新生治疗的潜在策略。然而,值得注意的是,由于抑癌基因PTEN受到抑制,这种促血管生成疗法可能存在诱导肿瘤发生的风险。因此,需要进一步研究以明确微小RNA治疗的安全性。除血管新生外,神经再生、突触发生和轴突重塑也在缺血性脑卒中神经功能恢复方面发挥着重要的作用,因此可能还有其他机制可能解释miR-181b的神经保护作用,这在未来的研究中有待探索。此外,PTEN/Akt信号通路下游的其他级联反应,是否也有助于缺血性脑卒中血管生成仍需进一步研究。
原文链接:https://doi.org/10.4103/1673-5374.367957
参考文献
[1] Xu X, Ge S, Jia R, et al. Hypoxia-induced miR-181b enhances angiogenesis of retinoblastoma cells by targeting PDCD10 and GATA6. Oncol Rep. 2015;33(6):2789-2796.
[2] Lv Y, Liu Z, Huang J, et al. LncRNA nuclear-enriched abundant transcript 1 regulates hypoxia-evoked apoptosis and autophagy via mediation of microRNA-181b. Mol Cell Biochem. 2020;464(1-2):193-203.
[3] Wen L, Wang YD, Shen DF, et al. Exosomes derived from bone marrow mesenchymal stem cells inhibit neuroinflammation after traumatic brain injury. Neural Regen Res. 2022;17(12):2717-2724.
[4] Xue L, Huang J, Zhang T, et al. PTEN inhibition enhances angiogenesis in an in vitro model of ischemic injury by promoting Akt phosphorylation and subsequent hypoxia inducible factor-1α upregulation. Metab Brain Dis. 2018;33(5):1679-1688.
[5] Geng W, Zhou G, Zhao B, et al. Liquiritigenin suppresses the activation of hepatic stellate cells via targeting miR-181b/PTEN axis. Phytomedicine. 2020;66:153108.

第一作者:薛丽霞,副主任医师,硕士生导师,中国卒中学会会员,中国抗癫痫协会会员,国际脑损伤协会(IBIA)会员。主持国家自然科学基金1项,参与国家自然科学基金2项,上海市浦江人才计划1项,发表SCI论著9篇,其中6篇为临床研究,主持ClinicalTrials.gov临床研究5项。研究方向:脑卒中、脑损伤等神经重症,癫痫、神经退行性疾病基础与临床。
基金支持:国家自然科学基金项目(81801169)

通讯作者:陈浩,副主任医师,硕士生导师,上海市医学会神经外科分会、创伤专科分会青年委员,中国医师协会周围神经专业委员会周围神经肿瘤专业组委员,美国神经外科医师学会(CNS)会员,国际神经外科教育与培训系列网课授课专家。主持国家自然科学基金2项、上海市浦江人才计划1项,发表SCI论著11篇,其中8篇为临床研究,3项临床成果被国内国际指南或专家共识所引用。研究方向:颅脑创伤、脑卒中等神经重症基础与临床;微创神经外科相关技术与器械研发;神经外科人工智能技术开发与应用。
基金支持:国家自然科学基金项目(82071404)





