脊髓损伤
-
Figure 1|Bioinformatics analysis identifies lncRNA Vof-16 enrichment in inflammation- and apoptosis-related pathways.
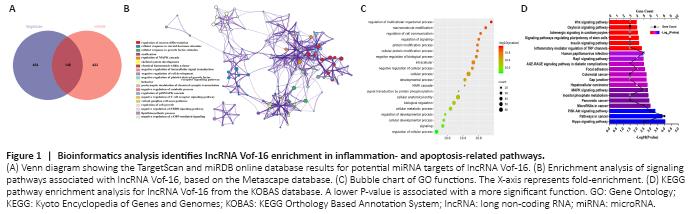
Using RNAInter and miRDB bioinformatics software, 42 miRNA families were predicted to bind to lncRNA Vof-16, of which 3 intersection miRNAs were identified by both programs, including rno-miR-346, rno-miR-205, and rno-miR-874-3p. A total of 877 proteins were predicted to interact with lncRNA Vof-16, and 148 proteins were identified by both the TargetScan and miRDB online databases as potentially interacting with lncRNA Vof-16 (Figure 1A). The STRING and Metascape websites were used to analyze the functional enrichment of the 148 identified target genes (Figure 1B–D). Figure 1B shows a network diagram of the potential KEGG signaling pathways associated with lncRNA Vof-16. The size of the pathway was determined by the number of molecular targets involved. Excluding ossification, the negative regulation of catabolic processes, and the lipid biosynthetic process, which signaling pathways with little relevance for SCI, a total of 13 core potential signaling pathways were identified (Figure 1B). The GO enrichment analysis results demonstrated that the likely target genes for lncRNA Vof-16 are involved in the regulation of multiple biological processes, such as macromolecule modifications, cell communication, and protein modification processes (Figure 1C). As shown in Figure 1D, signaling pathways such as the phosphoinositide 3-kinase (PI3K)–protein kinase B (Akt) signaling pathway, the mitogen-activated protein kinase (MAPK) signaling pathway, inflammatory mediator regulation of transient receptor potential (TRP) channels, and the advanced glycation endproducts signaling pathway were identified, which are primarily associated with inflammation and apoptosis (Nakajima et al., 2015; Zhang et al., 2015; Fu et al., 2018; Shimizu et al., 2018), may be the primary signaling pathways through which lncRNA Vof-16 exerts effects on the pathophysiology of SCI. These findings provide a novel perspective on the underlying mechanisms of lncRNA Vof-16, but further validation with experimental evidence remains necessary to explore the functions of lncRNA Vof-16 in SCI.
Figure 2|Changes in lncRNA Vof-16 expression affect neuronal survival and proliferation in vitro.

A series of analyses were performed to determine the cellular function of lncRNA Vof-16 using rat PC12 cells transfected with lncRNA Vof-16 overexpression or knockdown lentivirus. As shown in Figure 2A and B, cells were evaluated for live/dead staining and Ki67 staining, 1, 3, or 5 days after 72 hours of transfection. The live/dead staining showed an increase in the cell death rate for the lncRNA Vof-16 overexpression group compared with the control group (P < 0.001; Figure 2C). In contrast, we observed that cell viability was elevated in the lncRNAs Vof-16 knockdown group compared with that in the control group (P < 0.01; Figure 2C). Except for the overexpression group, Ki67 staining indicated that PC12 cell proliferation decreased in a time-dependent manner (P < 0.01; Figure 2D). The reduction in cell proliferation plateaued 5 days after transfection in the lncRNA Vof-16 knockdown group compared with the control group (P > 0.05; Figure 2D). These results suggested lncRNA Vof-16 knockdown increased the survival and proliferation of PC12 cells, whereas lncRNA Vof-16 overexpression was not conducive to cell survival and proliferation.
Figure 3|LncRNA Vof-16 affects cell migration and neurite outgrowth in PC12 cells.
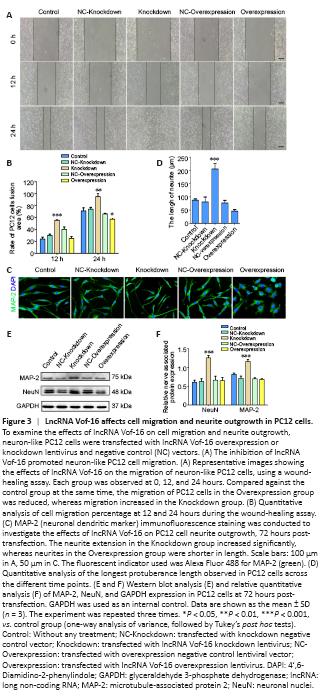
A wound-healing assay was used to detect the migration abilities of PC12 cells after changing lncRNA Vof-16 expression levels, in which the cell fusion area was used to represent the migration ability (Figure 3A). Images were acquired at 0, 12, and 24 hours. As demonstrated in Figure 3B, the wound-healing assay revealed a reduction in the migration ability of PC12 cells transfected with the lncRNA Vof-16 overexpression lentivirus compared with the control group, accounting for 57.21 ± 3.02% of the scratch area relative to the 0-hour point (P < 0.05 for the overexpression group). Moreover, the lncRNA Vof-16 knockdown group filled 55.5 ± 2.5% (P < 0.001) and 95 ± 6.25% (P < 0.01) of the wounded area after 12 and 24 hours relative to 0 hour, respectively, which was a significant increase compared with the control group (Figure 3B). These results demonstrated that lncRNA Vof-16 knockdown promoted cell migration, whereas the overexpression of lncRNA Vof-16 had the opposite effect.
We next examined the effects of lncRNA Vof-16 on neurite extension using staining for MAP-2, a neuronal dendritic marker (Shafit-Zagardo and Kalcheva, 1998) that is expressed in neuronal cell bodies and dendrites and plays an important role in nerve regeneration and network reconstruction (Figure 3C). The results showed that neurite extension in the lncRNA Vof-16 knockdown group increased significantly, by almost 2-fold (205.4 ± 22.57 μm) compared with the control group (Figure 3D), whereas treatment with the lentiviral negative controls had no significant effect on neurite outgrowth. However, the neurites in the lncRNA Vof-16 overexpression group were shorter (46.20 ± 5.79 μm) than those in the control group (Figure 3D). The results of western blot analysis were consistent with the immunocytochemical staining results (Figure 3E), demonstrating that the expression levels of NeuN, an excellent neuronal marker (Duan et al., 2016), and MAP-2 were significantly increased in the lncRNA Vof-16 knockdown group than in the control group (Figure 3F), indicating an increase in neurites. These data suggested that inhibiting the expression of lncRNA Vof-16 in PC12 cells was beneficial for neurite outgrowth.
Figure 4|LncRNA Vof-16 is associated with inflammation and apoptosis pathways in vitro.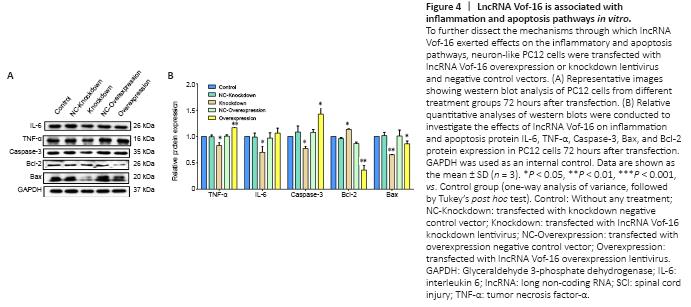
To assess the results of the bioinformatics analysis (Figure 1), the mechanisms through which lncRNA Vof-16 exerted effects on the inflammatory and apoptosis pathways during SCI were further dissected by using western blot to detect the expression levels of the inflammation-related proteins TNF-α and IL-6, the pro-apoptosis factors Bax and Caspase-3, and the anti-apoptosis protein Bcl-2 in PC12 cells. As shown in Figure 4, compared with the control group, the overexpression of lncRNA Vof-16 increased the levels of the inflammatory factors TNF-α (P < 0.01) and IL-6 (P > 0.05), enhanced the expression levels of the pro-apoptosis factor Caspase-3 (P < 0.05), and inhibited the expression of the anti-apoptotic factor Bcl-2 (P < 0.01), which indicated that the overexpression of lncRNA Vof-16 in PC12 cells enhanced inflammation and apoptosis. The knockdown of lncRNA Vof-16 showed the opposite trends (P < 0.05). Collectively, these results suggested that lncRNA Vof-16 influences neuroinflammation and apoptosis, contributing to a disadvantageous environment that aggravates the pathophysiology of SCI.
Figure 5|LncRNA Vof-16 is significantly upregulated and aggravated neuroinflammation and apoptosis after SCI.
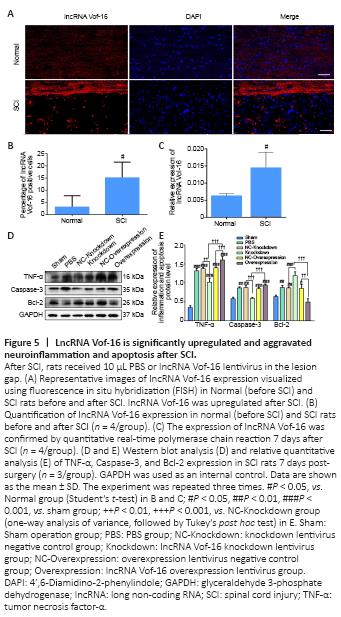
The expression of lncRNA Vof-16 in rats before SCI (Normal group) and after SCI (SCI group) was examined by FISH and RNA analysis (Figure 5). As shown in Figure 5A, lncRNA Vof-16 was differentially upregulated during the course of SCI. Compared with the Normal group, FISH quantification showed that the lncRNA Vof-16 expression level increased dramatically in the SCI group (Figure 5B). Similar results were observed with the qRT-PCR assay, in which high levels of lncRNA Vof-16 were observed in injured tissue derived from rats after 7 days post-SCI (P < 0.01 vs. the SCI group; Figure 5C).
To determine whether lncRNA Vof-16 affects biological behaviors through the regulation of inflammation and apoptosis pathways in vivo, western blot analysis was performed 7 days post-SCI in rats from different treatment groups. As shown in Figure 5D, the expression level of the pro-inflammatory cytokine TNF-α was reduced in the lncRNA Vof-16 Knockdown group, whereas the expression levels of TNF-α and Caspase-3 were upregulated and the level of Bcl-2 was downregulated in the lncRNA Vof-16 Overexpression group compared with the Sham group (Figure 5E). These results suggest that the upregulation of lncRNA Vof-16 after SCI plays a negative regulatory role by aggravating the local inflammatory microenvironment and inhibiting the survival of spinal cord neurons, whereas inhibiting the expression of lncRNA Vof-16 may improve the inflammatory microenvironment, promoting nerve regeneration and repair after SCI.
Figure 6|Local delivery of the lncRNA Vof-16 knockdown lentivirus is beneficial for neuronal survival following SCI.
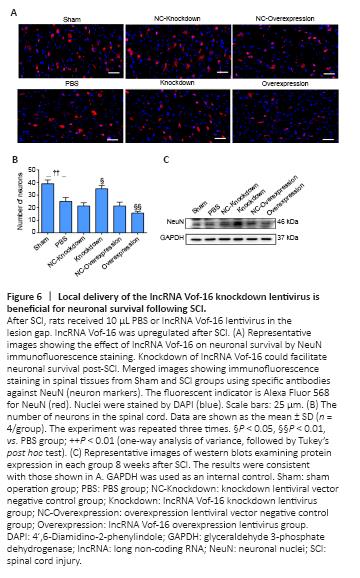
The loss and death of large numbers of neurons after SCI remains a major obstacle to functional recovery. To further investigate the effects of lncRNA Vof-16 lentivirus on spinal cord neurons in vivo, we used the neuron-specific antibody NeuN (Duan et al., 2016) to perform the immunofluorescence staining of spinal cord neurons (Figure 6A). As shown in Figure 6B, the number of neurons in the PBS group was significantly lower than that in the Sham group (25 ± 3 neurons/visual field, vs. 39 ± 3 neurons/visual field, P < 0.01), suggesting that a large number of neurons died and were lost after the spinal cord transection injury. In addition, compared with the Sham group, the number of neurons in the lncRNA Vof-16 Knockdown group increased significantly (35 ± 6.25 neurons/visual field, P < 0.05), whereas the number of neurons in the lncRNA Vof-16 Overexpression group decreased significantly (15.67 ± 1.53 neurons/visual field, P < 0.01). The western blot results also indicated that the downregulation of lncRNA Vof-16 expression in rats was associated with the promotion of spinal cord neuron survival, whereas the overexpression of lncRNA Vof-16 was associated with increased spinal cord neuron loss (Figure 6C).
Figure 7|Transplantation of lncRNA Vof-16 knockdown lentivirus promotes the outgrowth of regenerative fibers in rats with SCI.
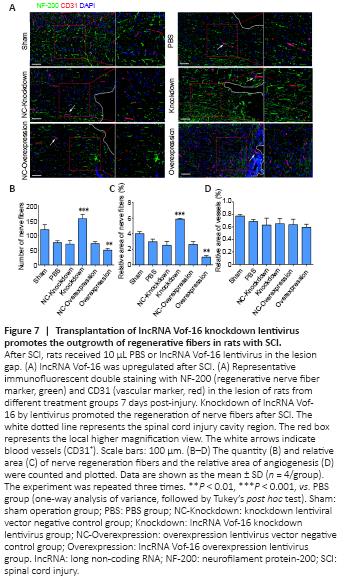
The nerve fiber regeneration in each group was investigated 8 weeks after SCI by immunofluorescence staining against the nerve fiber marker NF-200 and the vascular marker CD31 (Mohamadi et al., 2018) (Figure 7A). The results showed an increase in the linear distribution of cord-like nerve fibers in the spinal cord tissue of the Sham and lncRNA Vof-16 Knockdown groups, whereas the other groups presented fewer nerve fibers with scattered distributions (Figure 7B and C). These results suggested that the in vivo treatment with the lncRNA Vof-16 knockdown lentivirus was able to promote the regeneration of nerve fibers after SCI. In addition, the morphological analysis of CD31 immunofluorescence staining (vascular marker) preliminary showed that the blood vessels in the Sham group were primarily microvessels, based on vessel diameter, with a few vasodilating phenomena observed near the injury site after SCI (Figure 7D).
Figure 8|LncRNA Vof-16 knockdown improves functional recovery after SCI.
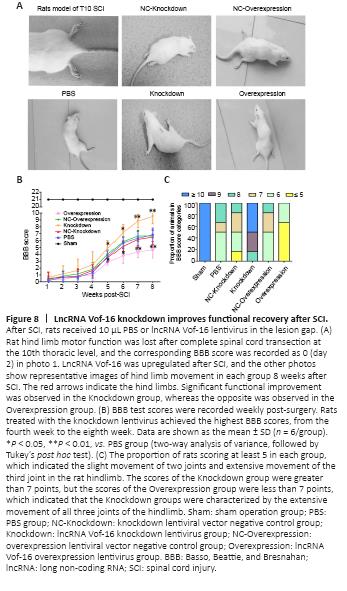
The effects of lncRNA Vof-16 on locomotor recovery after SCI were evaluated weekly using the BBB scale (Figure 8A). We found that all rats subjected to spinal cord contusions were paralyzed in both hind limbs starting from the second-day post-injury (Figure 8B). As shown in Figure 8B, hind limb locomotor activity improved gradually during the experimental period, as demonstrated by the increase in BBB scores. No significant differences in BBB scores were observed across the various SCI groups one week after SCI (Figure 8B). The rats in all SCI groups showed significant functional recovery at 4–8 weeks. However, the functional recovery in the lncRNA Vof-16 Overexpression group was remarkably slower than that for the other SCI groups (P < 0.01; Figure 8B), whereas the functional recovery in the lncRNA Vof-16 Knockdown group appeared to be faster than that of other groups starting 5 weeks post-surgery and became significantly improved starting at week 7 compared with the other SCI groups (P < 0.01; Figure 8B). By week 8, significant differences in the BBB scores were observed between the lncRNA Vof-16 Knockdown and Overexpression groups (8.8 ± 0.98 vs. 4.3 ± 8.2, P < 0.01). To further assess the effects of lncRNA Vof-16 on neurological recovery, the BBB scores were calculated for rats in each group 8 weeks after surgery. As shown in Figure 8C, the proportion of rats with a score less than or equal to 5 in the lncRNA Vof-16 Overexpression group reached 66.7%, but no animals in the groups without overexpression achieved this score. In contrast, the BBB scores in the lncRNA Vof-16 Knockdown group were all greater than 8, whereas only 16.7% of all animals in all groups except the knockdown group reached this level. These data indicated that the SCI model was established successfully and that the inhibition of lncRNA Vof-16 in SCI rats could improve the recovery of motor function.