颅神经损伤
-
Figure 2|MiR-29b increases glutamate-induced PC12 cell apoptosis.
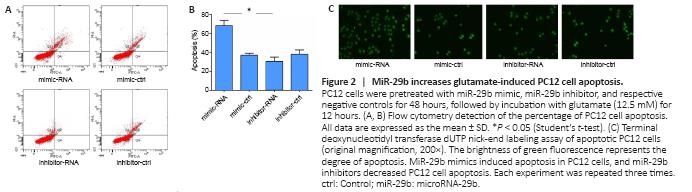
To further explore the effects of highly expressed miR-29b on the specific function of cerebral ischemic nerve cells, we overexpressed or inhibited miR-29b in PC12 cells and examined cell apoptosis. First, in preliminary experiments, we found that PC12 cells treated with miR-29b mimics or 24 hours showed the most obvious apoptosis characteristics (TUNEL-positive cells). Therefore, we chose the 24-hour time point for the following experiment. We used flow cytometry to detect apoptosis, and the results showed that miR-29b mimics increased the percentage of apoptosis induced by glutamic acid in PC12 cells (P < 0.05). The miR-29b inhibitor was able to reduce glutamate-induced apoptosis in PC12 cells (Figure 2A and B). We also used the TUNEL assay to detect apoptosis in PC12 cells and found that miR-29b mimics induced apoptosis in PC12 cells, whereas miR-29b inhibitors decreased PC12 cell apoptosis (Figure 2C). In summary, the above experimental results indicated that miR-29b overexpression in cerebral ischemic diseases could promote glutamate-induced apoptosis.
Figure 3|MiR-29b decreases SOD activity and increases MDA content in PC12 cells.
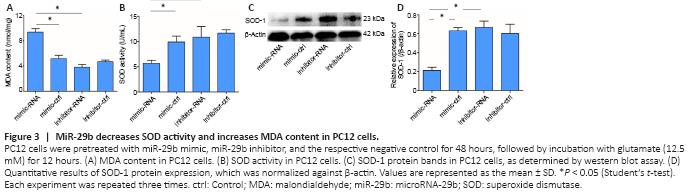
Oxidative stress is an important mechanism in cerebral ischemic diseases. SOD and MDA are key indicators used to evaluate the level of oxidative stress in cells (Li et al., 2017b). Therefore, we detected the expression of SOD and MDA in the ischemic brain tissue of mice and a cell model of cerebral ischemia in this study. The results showed that MDA [a lipid peroxidation marker (Tsikas, 2017)] level in PC12 cells treated with the miR-29b mimic was significantly increased compared with that in the control group (P < 0.05), while MDA level in PC12 cells treated with the miR-29b inhibitor was slightly decreased compared with that in the control group (P > 0.05; Figure 3A). The SOD activity of PC12 cells treated with miR-29b mimics was significantly decreased compared with that of the control group (P < 0.05), while the SOD activity of PC12 cells treated with miR-29b inhibitor was slightly decreased compared with that of the control group (P > 0.05; Figure 3B). We also examined the expression level of the oxidative stress-related protein SOD-1. The SOD-1 protein expression level in the miR-29b mimic-treated group was significantly lower than that of the control group, and the SOD-1 protein expression level in the miR-29b inhibitor-treated group was higher than that of the control group (all P < 0.05; Figure 3C and D). These results revealed that miR-29b might be involved in the regulation of oxidative stress in nerve cells under conditions of cerebral ischemic disease.
Figure 4|MiR-29b antagomir ameliorates the neurological deficits in cerebral ischemia model mice.
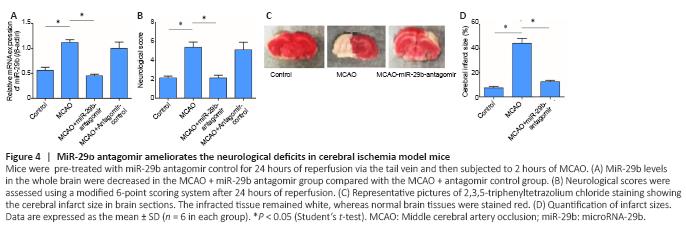
o further investigate the potential role played by miR-29b in cerebral ischemic diseases, we established a mouse cerebral ischemic disease model. We used an miR-29b antagomir to inhibit miR-29b expression in mice and then established a mouse cerebral ischemia model, followed by the detection of the neurological functions of mice after ischemia. The results showed that compared with the control group, the relative mRNA expression of miR-29b in the MCAO group was significantly increased (P < 0.05; Figure 4A). Neurological scoring was performed after 24 hours of cerebral ischemia in mice. The neurological score of the MCAO group was significantly increased compared with that in the control group and the neurological score of miR-29b-antagomir group was significantly lower than that of MACO group (P < 0.05; Figure 4B). We also found that the cerebral infarct size in the MCAO + miR-29b antagomir group was significantly decreased compared with that in the MCAO group (P < 0.05; Figure 4C and D). Therefore, these experiments indicated that inhibiting the expression of miR-29b in cerebral ischemic diseases may alleviate the neurological symptoms associated with cerebral ischemic diseases. No differences were observed between the MCAO + antagomir control group and the control group (P > 0.05); therefore, the MCAO + antagomir control group was not included in Figure 4.
Figure 5|MiR-29b antagomir inhibits apoptosis in cerebral ischemia model mice.
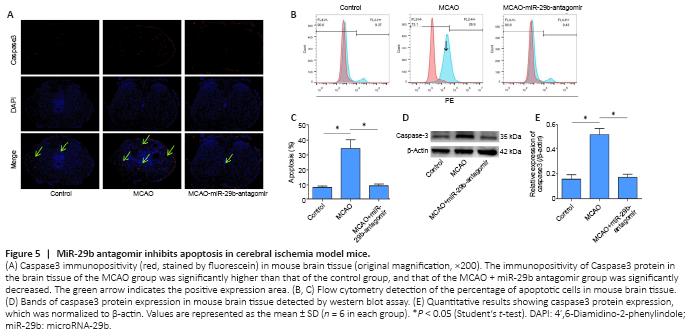
Caspase3 protein is a protein representing cell apoptosis. We used immunofluorescence staining to stain brain tissue sections for caspase3 protein. We found that the number of apoptotic cells in the brain tissue of mice treated with the miR-29b antagomir was significantly decreased compared with that in the MCAO group. The immunopositivity of Caspase3 protein in the brain tissue of the MCAO group was significantly higher than that of the control group, whereas the level of Caspase3 in the CAO-miR-29b-antagomir group was significantly decreased than that of the control group (Figure 5A). We removed the brain tissue from the mice and used flow cytometry to detect the percentage of apoptotic cells. We also found that the percentage of apoptotic cells in the MCAO + miR-29b antagomir group was significantly decreased compared with that in the MCAO group (P < 0.05; Figure 5B and C). We used the western blot assay to detect the expression of apoptotic proteins in the brain tissue of mice. Caspase3 protein expression in the MCAO + miR-29b antagomir group was significantly lower than that in the MCAO group (P < 0.05; Figure 5D and E). No significant difference was observed between the MCAO + antagomir control group and the control group (P > 0.05); therefore, the MCAO + antagomir control group was not included in Figure 5.
Figure 6|MiR-29b antagomir upregulates antioxidant systems in mouse brain tissues following cerebral ischemia.
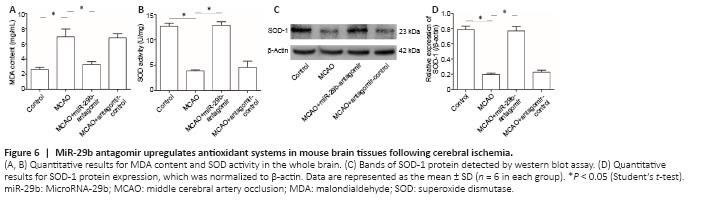
We examined the effects of miR-29b expression on the level of oxidative stress in the brain tissue of cerebral ischemic mice. The results showed that the MDA level in the brain tissue of the MCAO group was significantly increased compared with that of the control group (P < 0.05). The MDA level of mice in the MCAO + miR-29b antagomir group was significantly decreased compared with that in MCAO + antagomir control group (P < 0.05; Figure 6A). The SOD activity of the brain tissue of the MCAO group was significantly decreased compared with that of the control group, whereas the SOD activity of the MCAO + miR-29b antagomir group was significantly increased compared with that of the MCAO group (all P < 0.05; Figure 6B). Western blot analysis was used to detect the expression of SOD-1 in the brain tissue of mice, which revealed that the level of SOD-1 protein in the brain tissue of the MCAO group was lower than that of the control group (P < 0.05). The level of SOD-1 protein in the MCAO + miR-29b antagomir group was significantly higher than that in the MCAO + antagomir control group (P < 0.05; Figure 6C and D).
Figure 7|MiR-29b regulates the PI3K/Akt/Bax signaling pathway in MCAO model mice.
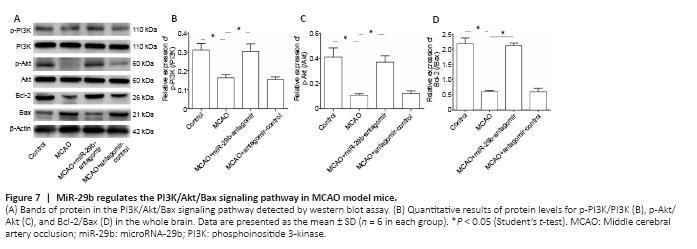
To explore the specific mechanisms through which miR-29b regulates cerebral ischemic cell apoptosis, we further examined changes in apoptosis-related pathways. We examined the PI3K/Akt/Bax signaling pathway in MCAO mice by western blot analysis. The miR-29b antagomir treatment promoted the activation of the PI3K/Akt signaling pathway in the brain tissue of cerebral ischemic mice (Figure 7A–C). Treatment with the miR-29b antagomir promoted the expression of the apoptotic protein Bax and inhibited the expression of the anti-apoptotic protein Bcl-2 (Figure 7A and D). These results suggested that miR-29b may promote apoptosis and oxidative stress levels in the brain tissues of cerebral ischemic mice by regulating the PI3K/Akt/Bax signaling pathway.