神经退行性病
-
Figure 1|SH-SY5Y-derived neurons for studying TAU trafficking and tauopathy-related TAU pathology.
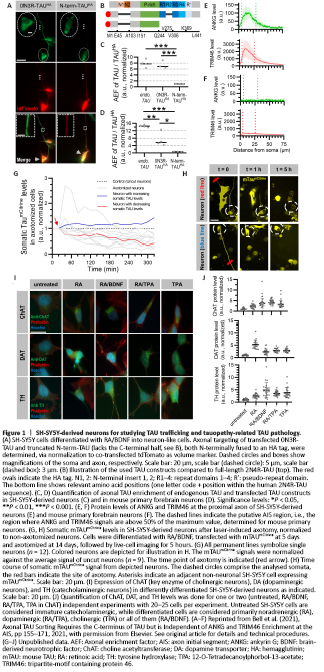
TAU-intrinsic factors involved in TAU trafficking: We recently discovered that SH-SY5Y-derived neurons sort endogenous TAU with a slightly lower efficiency than mouse primary neurons and iPSC-derived neurons. However, SH-SY5Y-derived neurons show and tolerate transient overexpression of transfected TAU much longer and achieve endogenous-like sorting efficiency, in contrast to primary rodent neurons (Figure 1A–D) (Bell et al., 2021). This enables us to study TAU trafficking of truncated, modified or otherwise engineered TAU constructs. Initial data with a truncated C-terminus-lacking TAU construct show similar sorting behaviour in SH-SY5Y-derived neurons, mouse primary neurons and iPSC-derived neurons (Bell et al., 2021). Hence, we consider a comprehensive analysis of domains, motifs, or interaction sites required for successful sorting feasible in SH-SY5Y-derived neurons.
Cellular and axon initial segment-specific factors involved in TAU trafficking: The axon initial segment (AIS), a highly specialized region at the proximal axon with ankyrin G (ANKG) as a master organizer (Rasband, 2010), is critical for developing neuronal polarity and action potential generation. In rodent primary neurons, ANKG or the tripartite motif-containing protein 46 (TRIM46) were critical for successful axonal TAU sorting (Rasband, 2010; Van Beuningen et al., 2015). Surprisingly, SH-SY5Y-derived neurons show efficient TAU sorting without any detectable accumulation of ANKG and TRIM46 at the proximal axon (Figure 1E and F). This lack of a classical AIS in SH-SY5Y-derived neurons could be the result of neuronal immaturity (primary rodent neurons develop TRIM46/ANKG accumulation at DIV3/4) and has to be considered as a potential limitation of mimicking the in vivo situation. However, this cell system bears potential for future studies, like addressing the importance of TRIM46-mediated MT polarization for TAU trafficking, and the general necessity of TRIM46 or ANKG for MT polarization at the AIS.
The (largely) ANKG/TRIM46-independent TAU sorting hints at the presence of unidentified mediators of neuronal (and TAU) polarity. Recent peroxidase- or biotinylation-based proximity labelling methods could be helpful to assess TAU-AIS interactions in SH-SY5Y-derived neurons, also in comparison with other neuronal cell models (Cho et al., 2020). However, AIS-specific proximity labeling requires high sensitivity to detect transient interactions and a system enabling site-specific labelling without affecting the TAU trafficking process.
Synaptotoxicity and spine loss due to TAU missorting: Elevated levels of dendritic TAU result in mitochondrial mislocalization and postsynaptic spine loss via TAU-induced recruitment of the excitotoxicity-mediating kinase Fyn, or tubulin tyrosine ligase-like proteins that induce microtubule breakdown (Ittner and Ittner, 2018). The cascade from TAU missorting to spine degradation, however, is under debate. Suitable neuronal cell models must exhibit functional synapses and dendritic spine formation.
In SH-SY5Y-derived neurons, different pre- and postsynaptic markers as well as vesicle proteins are expressed (Bell and Zempel, 2021). However, the spatial distribution of these markers along axonal or dendritic processes does not faithfully recapitulate the in vivo situation. Robust co-localization of pre- and postsynaptic markers like seen in rodent primary neurons or in iPSC- and NPC-derived neurons is not observed. The obvious limitation of SH-SY5Y-derived neurons is the short lifespan of the cultures, with maximum growth periods of four to five weeks after RA/BDNF treatment. Thus, despite the reported excitability and the presence of functional synaptic vesicles in SH-SY5Y-derived neurons (Bell and Zempel, 2021), these cells might lack the degree of maturity that is necessary to mimic the synaptotoxic effects of pathological TAU in disease-burdened human neurons.While many features of tauopathies like TAU missorting, hyperphosphorylation, or postsynaptic degradation can be induced in cell culture systems with external stressors, the formation of insoluble TAU aggregates is not inducible in most systems, including SH-SY5Y cells. Only with overexpression of pro-aggregant TAU mutants, TAU aggregates or inclusions can be obtained (Figure 1A, right panels). However, this artificial way of TAU aggregation, also performed in SH-SY5Y cells (Bell and Zempel, 2021), may generate aggregates reminiscent of certain genetic forms of tauopathy, but different from insoluble TAU aggregates found in AD brains.
Strikingly, NPC-derived neurons were generated that successfully developed profound amyloid-β (Aβ) pathology, Aβ-induced TAU aggregation, and AD brain-like neuronal morphology after two to three months of cultivation (Choi et al., 2014). This AD-like pathology was achieved with lentiviral transduction of amyloid precursor protein and presenilin variants known from familial AD cases, and by using a three-dimensional Matrigel-based culture matrix. This indicates that NPC- or iPSC-derived neuron cultures are more promising for studying TAU aggregation, in principle due to the much higher culture life span, and their demonstrated ability to form aggregates composed of endogenous physiological TAU as seen in AD patients.
Neuronal subtype-specific susceptibility for TAU pathology: In many tauopathies including AD, the progression of TAU pathology is brain region-specific, e.g. the locus coeruleus is usually affected very early and even in asymptomatic individuals. This suggests that specific inter-neuronal differences are critical for the susceptibility to TAU pathology. However, the underlying neuron subtype-specific features remain enigmatic.
For SH-SY5Y-derived neurons, the reported neuronal identity include, depending on the differentiation treatment and the analysed biochemical markers, primarily noradrenergic, dopaminergic, or cholinergic neuronal subtypes (Kovalevich and Langford, 2013). Interestingly, these neuron types are found in subcortical nuclei that are early affected in many tauopathies including AD: the locus coeruleus, the nucleus basalis, and the substantia nigra pars compacta. Since the underlying pathomechanisms are still unclear, steerable generation of distinct SH-SY5Y-derived neuron subtypes would allow to study TAU-based toxicity in different neuronal subpopulations.
However, the reported identity of SH-SY5Y-derived neurons after specific differentiation procedures is inconsistent. Hence, the neuronal identities of SH-SY5Y-derived neurons might be not distinctive cellular subtypes, but rather accentuations of a spectrum of the same entity (Bell and Zempel, 2021). We conducted comparative analyses of four differentiation procedures but did not observe clearly distinct expression patterns of key enzymes commonly used to define neuronal subtypes (Figure 1I and J). Another principle obstacle is that general age-related changes in the cellular functionality, and major features of locus coeruleus, nucleus basalis or substantia nigra pars compacta neurons thought to have large impact on their increased susceptibility are certainly difficult to recapitulate in cell culture (Bell and Zempel, 2021).
Taken together, the resulting neuronal identities are ill-defined and might not recapitulate sufficient features of cells affected in AD and related tauopathies to be useful for studying neuronal subtype-specific susceptibility to TAU pathology.
Traumatic brain injury modelling with induced axon lesion: In one subgroup of tauopathies, including traumatic brain injury and chronic traumatic encephalopathy, mechanically evoked traumatic axon injury (TAI) precedes TAU pathology and NFT formation (Blennow et al., 2012). The underlying pathological cascade is still under debate. Although TAI mouse models exist, an in vitro laser-inducible axotomy cell model bears potential for several approaches. It allows the induction of precise lesions on a single-cell and even compartment-specific scale. Available live-cell imaging tools (e.g., photoconvertible TAU constructs, live AIS cytoskeleton markers and biosensors) would allow to monitor TAI-dependent alterations of TAU trafficking, phosphorylation, or the AIS architecture.
We tested whether SH-SY5Y-derived neurons are suitable for UV laser-induced axotomy. For this, we measured the somatic levels of transfected mTAUmCitrine after axotomy, in comparison to uncut neurons (Figure 1G and H). Unfortunately, many neurons detached several hours after axotomy, impeding downstream analyses. Of note, preliminary experiments with primary mouse neurons and iPSC-derived neurons suggested a higher degree of attachment and viability (data not shown).