脑损伤
-
Figure 1|Hypoxic-ischemic encephalopathy is one of the main causes of mortality and disabilities in newborns, and therapeutic hypothermia (TH) is the only standard clinical treatment used to date.
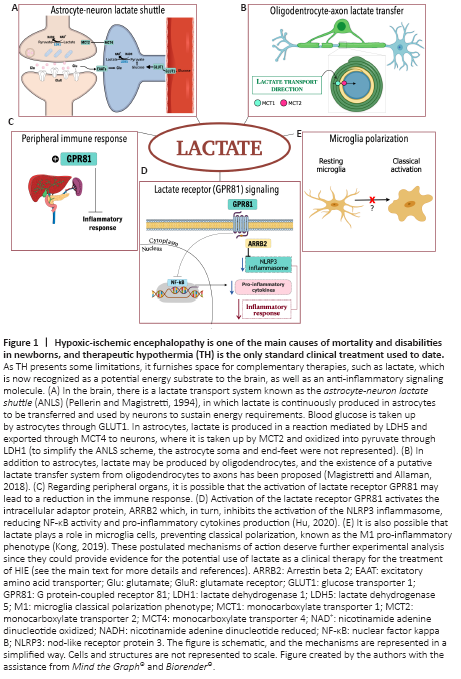
This shuttling system was first reported in skeletal muscle cells (Brooks, 1985). However, there is compelling evidence that there is a similar system between CNS cells (Pellerin and Magistretti, 1994; Magistretti and Allaman, 2018; Figure 1A). Briefly, ANLS allows cells with a high NADH:NAD+ ratio, such as astrocytes, to support oxidative cells with a lower NADH:NAD+ ratio, such as neurons, via lactate exportation, ensuring mitochondrial adenosine triphosphate (ATP) production during neuronal activation under physiological or pathological conditions. Lactate is produced in a redox reaction mediated by isoform 5 of enzyme lactate dehydrogenase (LDH5, abundant in glycolytic cells, such as astrocytes, with high amounts of reducing equivalents, i.e., NADH), guaranteeing the regeneration of NAD+, which is important to glycolytic flux maintenance. Lactate is exported through MCT4 from astrocytes to neurons, in which it is taken up by MCT2 and oxidized into pyruvate, through LDH1. This mechanism is useful for neurons suffering from oxygen deprivation, since importing lactate means that neurons do not need to use two ATP molecules in the investment phase of glycolysis (Magistretti and Allaman, 2018).
The existence of machinery to oxidize lactate in neurons and the presence of transporters that allow lactate to cross the BBB in the neonatal brain reinforces the idea of using lactate administered peripherally as a possible neuroprotective agent following hypoxia-ischemia.
A route into the CNS: Neonatal hypoxia-ischemia leads to a transient opening of the BBB (Lee et al., 2017). While this process allows the entrance of cytokines and large molecules into the CNS, which contributes to the extent of brain damage, it may also enable therapeutic molecules to reach different compromised areas of the brain (Lee et al., 2017). The post-HI enhancement of BBB permeability for lactate, may be due to the high expression of MCTs in the developmental brain (Pierre and Pellerin, 2005) or BBB disruption following an HI event (Lee et al., 2017). Accordingly, an understanding of the time-frame when BBB permeability is increased may streamline the delivery of therapeutic molecules for neuroprotection following an HI event. Thus, evaluating lactate uptake and transport through and in view of increased BBB permeability would provide new insights into HIE treatments. These analyses could uncover different therapies for the management of HIE, including lactate.
Lactate as an energy booster: ATP deficiency stimulates lactate production in astrocytes and oligodendrocytes (Magistretti and Allaman, 2018). Furthermore, lactate has neuroprotective effects in pathological conditions, such as brain ischemia and psychiatric disorders in adult mice and traumatic brain injury in humans (Magistretti and Allaman, 2018). There are only two studies in the literature evaluating the effect of lactate therapy on HIE harmful outcomes, both of which studied a rat model with neonatal HI (Roumes et al., 2021; Tassinari et al., 2020). Neither of these studies evaluated MCTs expression; however, peripheral administration of lactate increased lactate levels in the hypothalamus (Tassinari et al., 2020) and allowed the 13C of 13C-lactate to be incorporated in brain metabolites in both hemispheres (Roumes et al., 2021). Altogether, these findings suggest that exogenous lactate was taken up and utilized by the neonatal brain, which showed high levels of MCTs (Pellerin et al., 1998). Therefore, we presume that ANLS is greatly involved in neuroprotection, which was observed after exogenous lactate administration (Roumes et al., 2021; Tassinari et al., 2020). Moreover, when lactate metabolism was inhibited through the LDH inhibitor oxamate, neuroprotection was not observed, suggesting that protection depends on lactate metabolism (Roumes et al., 2021).
Moreover, since the harmful effects of neonatal hypoxia are systemic, organs other than the brain could take advantage of the glucose-lactate shunt to fulfill energy requirements as the lactate-shuttle has already been described in skeletal muscle (Brooks, 1985), the heart, liver, and kidneys (Pierre and Pellerin, 2005). Although there is no detailed investigation into these aspects in neonates, this would be one more advantage of lactate administration after neonatal HIE.
Damage to the white matter: HIE also damages the white matter, which is plentiful in oligodendrocytes, the myelin-producer cells of the CNS. These cells are injured due to excitotoxicity and oxidative stress caused by HIE, and white matter damage can lead to mental and motor detriments. Nevertheless, oligodendrocytes may utilize lactate as an energy substrate (Pierre and Pellerin, 2005) and precursor for lipid synthesis enrolled in myelin sheet formation. Likewise, energy deprivation can cause a delay in myelinization and degeneration of white matter structures. This process constitutes an elementary feature in motor deficits found in cerebral palsy and other neurological disabilities (Gunn and Thoresen, 2019). Similar to ANLS, oligodendrocytes appear to present the machinery required to produce and export lactate through MCT1, which is rapidly taken up from axons through MCT2. The existence of a putative lactate transfer from oligodendrocytes to active axons has also been proposed (Magistretti and Allaman, 2018; Figure 1B).
Due to the presence of MCTs in astrocytes, oligodendrocytes, and neurons, ANLS and axo-myelinic transmission constitute the foremost, tightly regulated mechanism to fulfill neurons’ energy demands, and lactate is produced in and exported from these cells. Nevertheless, white matter maturation in neonates is still a matter of debate, but it could be another therapeutic target for lactate and deserves further investigation in the neonatal brain.
Anti-inflammatory effects of lactate: Elevated plasma lactate levels during HIE treatment may also produce peripheral effects related to the reduction of brain injury, such as attenuating immune response (Hu et al., 2020; Figure 1C). Indeed, it has been demonstrated that lactate treatment in a cell line of macrophages and a model of acute pancreatitis and hepatitis causes an immunomodulatory response via G protein-coupled receptor 81 (GPR81), also known as hydroxy-carboxylic acid receptor 1 (For review, see Hu et al., 2020; Figure 1D). Lactate reduces inflammasomes’ activation, especially that of nod-like receptor protein 3 (NLRP3), and consequently, the production of pro-inflammatory cytokines is also reduced via GPR81 signaling (Hu et al., 2020). Moreover, lactate interferes in classical microglial polarization induced by lipopolysaccharide in vitro and in vivo, reducing neuroinflammatory parameters and sickness behavior (Kong et al., 2019). Since lactate appears to play a role in the immune system, it could mediate microglial polarization (Figure 1E). Therefore, immune organs, such as the spleen, thymus, and liver, require further investigation in the context of lactate employment to reduce peripheral and central inflammation.