脑损伤
-
Figure 1|CircRNAs and ZO-1 expression changes after TBI.
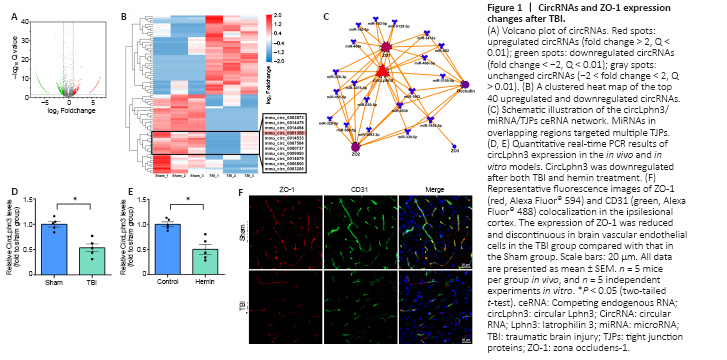
To determine the expression status of circRNAs, we selected three pairs of normal brain tissues and TBI tissues and conducted RNA-seq. The results showed 2015 circRNAs with 2-fold differential expression (Q < 0.01, Figure 1A), including 1336 downregulated and 679 upregulated circRNAs. The clustered heat map in Figure 1B shows the top 40 upregulated and downregulated circRNAs. RNAhybrid, miRanda, and TargetScan (Additional Table 1) were subsequently applied to predict the target miRNAs of all 40 differentially expressed circRNA. mmu_circ_0001358 (circLphn3) showed significant expression differences between the TBI and Sham groups, and the prediction showed that many circLphn3-targeted miRNAs were closely related to the TJP genes. In combination with the Starbase database, we found 19 miRNAs (including miR-185-5p) with potential circLphn3-binding sites. Among them, 14 miRNA had potential binding sites for ZO-1 mRNA, 11 miRNAs had binding sites for ZO-2 mRNA, 1 miRNA had binding sites for ZO-3 mRNA, and 8 miRNAs had binding sites for occludin mRNA (Figure 1C). We named mmu_circ_0001358 circLphn3 because of its host gene Lphn3. The average expression (FPKM) of circLphn3 was 1.923 in the Sham group compared with 0.457 in the TBI group, with a –4.2-fold change. Consistently, quantitative real-time PCR indicated that the expression level of circLphn3 in the injured brain tissue was significantly lower than that in the normal brain tissue (P < 0.05, Figure 1D). The in vitro experiment further indicated that the expression of circLphn3 was downregulated after cells were treated with 40 μM hemin for 12 hours (P < 0.05, vs. control group; Figure 1E). Furthermore, immunofluorescence photographs demonstrated colocalization of ZO-1 and CD31, showing loss and disruption of TJPs in brain vascular endothelial cells after TBI (Figure 1F).
Figure 2| Identification of circLphn3 as a circular RNA.

CircLphn3 was differentially expressed after TBI (Figure 1B). Using a genome browser (UCSC Date, http://genome.ucsc.edu/), we found that circLphn3 (1007 bp) is formed by exons 6–9 of Lphn3 (Figure 2A). Quantitative real-time PCR analysis showed that circLphn3 was amplified by divergent primers with cDNA as the template, but not with genomic DNA (Figure 2B). Additionally, quantitative real-time PCR showed that circLphn3 resisted RNase R, while its host gene, Lphn3 mRNA, was degraded by RNase R (Figure 2C and D). Sanger sequencing of the quantitative real-time PCR products amplified by circLphn3 divergent primers also confirmed a back-spliced junction in circLphn3 (Figure 2A). Moreover, the Cy3-circLphn3 FISH probe (Figure 2E) and cytoplasm/nucleus fraction isolation (Figure 2F) revealed that circLphn3 is primarily expressed in the cytoplasm.
Figure 3|CircLphn3 functions as a sponge for miR-185-5p and upregulates tight junction genes.
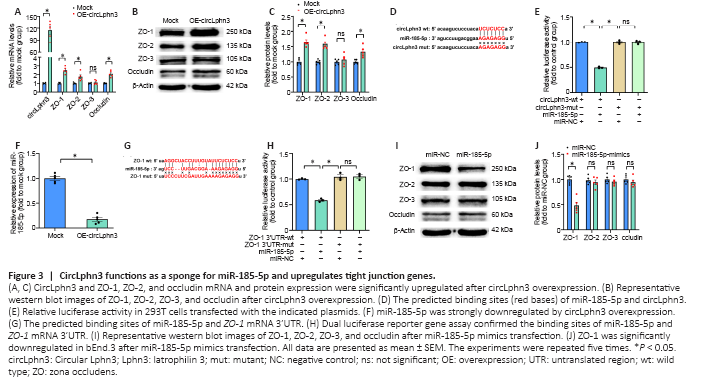
Figure 4|CircLphn3 protects BBB from hemin insult.

To investigate the effect of circLphn3 on the expression of ZO-1, ZO-2, ZO-3, and occludin, we transfected a circLphn3 overexpression plasmid into bEnd.3 cells for 24 hours and examined their mRNA and protein expression by quantitative real-time PCR and western blotting, respectively. The results showed that the mRNA levels of ZO-1, ZO-2, and occludin significantly increased after circLphn3 overexpression compared with those in the control group (P < 0.05; Figure 3A). In contrast, the mRNA levels of ZO-3 did not significantly change (P > 0.05, vs. Mock group; Figure 3A). Furthermore, circLphn3 overexpression increased the protein expression levels of ZO-1, ZO-2, and occludin (P < 0.05, vs. Mock group), but had no effect on ZO-3 in bEnd.3 cells (P > 0.05, vs. Mock group; Figure 3B and C).
To further verify the targeting of miR-185-5p by circLphn3, we transfected the psiCHECK2-circLphn3-wt or psiCHECK2-circLphn3-mut plasmid and miR-185-5p mimics into 293T cells for the luciferase reporter gene experiment. The results showed that the luciferase activity after co-transfection of miR-185-5p mimics with psiCHECK2-circLphn3-wt was lower than that of the control group (P < 0.05; Figure 3D and E). However, the luciferase activity after co-transfection of miR-185-5p mimics with psiCHECK2-circLphn3-mut was not significantly different from that of the control group (P > 0.05, Figure 3E). Further analysis showed that overexpression of circLphn3 significantly downregulated the expression of miR-185-5p in bEnd.3 cells (P < 0.05, vs. Mock group; Figure 3F). These results indicate that circLphn3 may act as a miR-185-5p sponge to downregulate the expression of miR-185-5p in bEnd.3 cells.The dual-luciferase reporter system indicated that the luciferase activity after co-transfection of miR-185-5p mimics with psiCHECK2-ZO1 3′UTR-wt was lower than that of the control group (P < 0.05; Figure 3G and H). In contrast, the luciferase activity after co-transfection of miR-185-5p mimics with psiCHECK2-ZO1 3′UTR-mut was not significantly different from that of the control group (P > 0.05; Figure 3H). Namely, miR-185-5p significantly inhibited the luciferase activity of the reporter gene containing the wild type ZO-1 mRNA 3′UTR (P < 0.05, vs. miR-NC group; Figure 3H), but not the mutant isoform (P > 0.05, vs. miR-NC group; Figure 3H). Additionally, our result showed that miR-185-5p mimics reduced the protein level of ZO-1 (P < 0.05, vs. miR-NC group), but had no effect on ZO-2, ZO-3, and occludin protein expression in bEnd.3 cells (P > 0.05, vs. miR-NC group; Figure 3I and J). These data confirmed that Tjp1 is a direct target of miR-185-5p. Furthermore, transfection with miR-185-5p mimics partly reversed the circLphn3-induced upregulation of ZO-1 protein expression (P < 0.05, vs. hemin + OE-circLphn3 group; Figure 4A and B). Importantly, transfection with miR-185-5p mimics reduced the protective effect of circLphn3 on hemin-induced cell permeability (P < 0.05, vs. hemin + OE-circLphn3 group; Figure 4C). These results further confirmed that circLphn3 reduces cell damage by modulating the miR-185-5p/ZO-1 axis, thus suggesting that circLphn3 may be a new target for protecting against subsequent BBB damage.
Next, we examined the function of circLphn3 in bEnd.3 cell dysfunction caused by hemin treatment. Hemin administration for 12 hours significantly decreased the expression of all four TJPs (including ZO-1, ZO-2, ZO-3, and occludin) in bEnd.3 cells (P < 0.05, vs. control group; Figure 4A and B). As shown in Figure 1E, the circLphn3 expression decreased in bEnd.3 cells treated with hemin (40 μM). To further explore the key role of circLphn3 in BBB damage, we established a monolayer bEnd.3 BBB model in the cell inserts for the permeability experiment. Samples were collected from the lower chamber at 1, 4, 12, 24, and 36 hours after hemin addition. The leakage of LY revealed that overexpression of circLphn3 decreased the hemin-induced high LY permeability at 24 and 36 hours (P < 0.05, vs. hemin group), while miR-185-5p transfection increased the permeability from 1 hour (P < 0.05, vs. hemin group; Figure 4C). Finally, ZO-1 immunofluorescence in bEnd.3 cells showed that overexpression of circLphn3 enhanced ZO-1 expression after hemin treatment, whereas miR-185-5p transfection attenuated the protective effect of circLphn3 on ZO-1 expression (Figure 4D).
点击此处查看全文