神经退行性病
-
Figure 1|MPP+ induced changes in HOTTIP/miR-615-3p/FOXO1 expression in SH-SY5Y and BV2 cells.

We treated SH-SY5Y and BV2 cells with different doses of MPP+ for 24 hours to investigate the role of HOTTIP, miR-615-3p and FOXO1 in PD. We conducted RT-PCR and western blot to measure HOTTIP, miR-615-3p and FOXO1 levels in the cells. MPP+ dose-dependently elevated HOTTIP expression and dampened miR-615-3p expression in SH-SY5Y and BV2 cells (Figure 1A–D). RT-PCR and western blot showed that the mRNA and protein expression of FOXO1 were elevated by MPP+, and the elevation was enhanced with the increasing MPP+ doses (Figure 1E–H). Thus, HOTTIP, miR-615-3p and FOXO1 were altered in MPP+-treated SH-SY5Y and BV2 cells.
Figure 2|The role of HOTTIP/miR-615-3p in regulating MPP+-induced neuronal toxicity in SH-SY5Y cells.

We transfected SH-SY5Y cells with vectors to overexpress HOTTIP, miR-615-3p, or both. Upregulating HOTTIP inhibited miR-615-3p, and upregulating miR-615-3p inhibited HOTTIP (Figure 2A and B). Next, the Cell Counting Kit-8 method and flow cytometry were used to examine cell viability and apoptosis, respectively. Compared with the control group, the HOTTIP group had reduced cell viability and increased cell apoptosis (Figure 2C and D). In contrast, miR-615-3p overexpression did not have a significant effect on cell viability and apoptosis. However, forced miR-615-3p overexpression in the HOTTIP group enhanced cell viability and reduced apoptosis (P < 0.05, vs. HOTTIP group; Figure 2C and D). Next, we treated SH-SY5Y cells with MPP+ to investigate the effect of HOTTIP and miR-615-3p on neuronal toxicity in the MPP+ PD cellular model. MPP+ treatment significantly decreased SH-SY5Y viability (P < 0.05, vs. control group; Figure 2E) and increased the apoptosis rate (P < 0.05, vs. control group; Figure 2F–G). In addition, miR-615-3p overexpression enhanced SH-SY5Y cell viability and suppressed apoptosis compared with the MPP+ group, whereas HOTTIP overexpression had the opposite effect, indicating that HOTTIP exacerbated the MPP+-induced cellular damage (P < 0.05; Figure 2E–G). Interestingly, the MPP+ + miR + lnc group had increased cell viability and decreased apoptosis compared with the MPP+ + lnc group, suggesting that miR-615-3p alleviated HOTTIP-mediated cellular damage. Furthermore, we measured the expression of apoptosis-related proteins (cleaved Caspase-3, Bax, and Bcl2) and NLRP3-ASC-cleaved Caspase-1 inflammasomes with western blot. MPP+ enhanced Caspase-3, Bax, and NLRP3-ASC-Caspase-1 inflammasome expression and suppressed Bcl2 expression (Figure 2H and I). miR-615-3p overexpression attenuated the proapoptotic proteins and NLRP3-ASC-Caspase-1 inflammasomes, whereas HOTTIP had the opposite effect (Figure 2H and I). The above results demonstrated that HOTTIP exacerbated the MPP+-induced neuronal toxicity, and miR-615-3p attenuated the MPP+-induced neuronal toxicity and weakened the effect of HOTTIP upregulation.
Figure 3|The function of HOTTIP/miR-615-3p in regulating MPP+-mediated inflammation in microglia (BV2 cells).
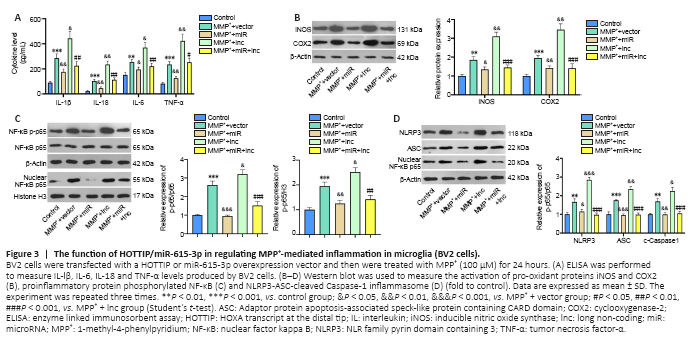
Microglial activation is a key feature in PD (Vivekanantham et al., 2015). Therefore, we constructed HOTTIP and miR-615-3p overexpression models in BV2 cells. ELISA and western blot showed that the proinflammatory cytokines (including IL-lβ, IL-6, IL-18, and TNF-α), the pro-oxidant proteins iNOS and COX2, and the proinflammatory protein phosphorylated NF-κB produced by BV2 cells were upregulated after MPP+ treatment (P < 0.05; Figure 3A–C). Overexpression of miR-615-3p and HOTTIP suppressed and enhanced the above-mentioned inflammatory response, respectively (P < 0.05; Figure 3A–C). BV2 cells in the MPP+ + miR + lnc group had a lower proinflammatory response than those in the MPP+ + lnc group (P < 0.05; Figure 3A–C). Next, we measured the NLRP3-ASC-Caspase-1 inflammasome expression in BV2 cells. MPP+ induced NLRP3-ASC-Caspase-1 inflammasome activation and miR-615-3p overexpression attenuated this effect (compared with the MPP+ group). In contrast, HOTTIP overexpression enhanced the NLRP3-ASC-Caspase-1 inflammasome activation compared with the MPP+ group (P < 0.05; Figure 3D). Moreover, miR-615-3p suppressed the HOTTIP-induced activation of NLRP3-ASC-Caspase-1 inflammasomes (P < 0.05; Figure 3D). These results suggest that HOTTIP was proinflammatory, whereas miR-615-3p was anti-inflammatory in MPP+-induced inflammation.
Figure 4|Both HOTTIP and FOXO1 target miR-615-3p in SH-SY5Y and BV2 cells.
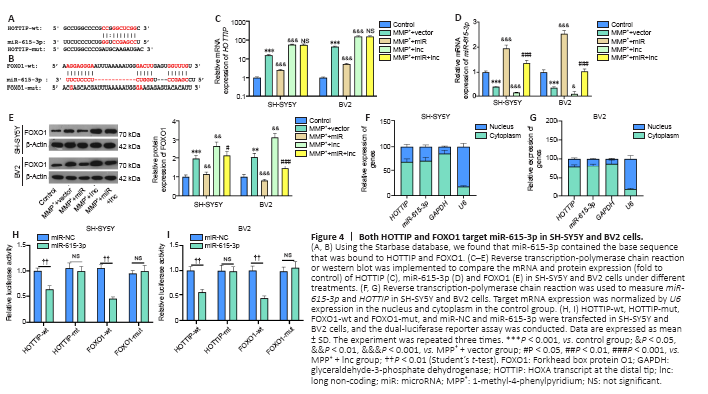
The online database Starbase (http://starbase.sysu.edu.cn/) was used to predict the potential targets of HOTTIP and FOXO1. Starbase indicated that miR-615-3p binds to HOTTIP and FOXO1 (Figure 4A and B). Next, we compared the profiles of miR-615-3p, HOTTIP and FOXO1 in SH-SY5Y and BV2 cells under different treatments. Compared with the MPP+ + vector group, the MPP+ + lnc group had increased HOTTIP and FOXO1 levels, and decreased miR-615-3p levels (Figure 4C–E). In contrast, FOXO1 was downregulated, miR-615-3p was upregulated, and HOTTIP did not change significantly in the MPP+ + lnc + miR group compared with the MMP+ + lnc group (Figure 4C–E). Next, we implemented RT-PCR to examine the distribution of miR-615-3p and HOTTIP in SH-SY5Y and BV2 cells. Both HOTTIP and miR-615-3p were mainly distributed in the cytoplasm (Figure 4F–G). Furthermore, we carried out a dual-luciferase reporter assay to examine the association between HOTTIP and FOXO1. The miR-615-3p mimics markedly dampened the luciferase activity of cells transfected with HOTTIP-wt and FOXO1-wt (containing miR-615-3p binding sites), and had little impact on that of cells transfected with HOTTIP-mut and FOXO1-mut (containing mutated miR-615-3p binding sites) (Figure 4H–I). Thus, HOTTIP inhibited miR-615-3p, and the latter targeted the 3′-untranslated region of FOXO1 in SH-SY5Y and BV2 cells.
Figure 5|FOXO1 overexpression exacerbates MPP+-induced neuronal damage in SH-SY5Y cells.
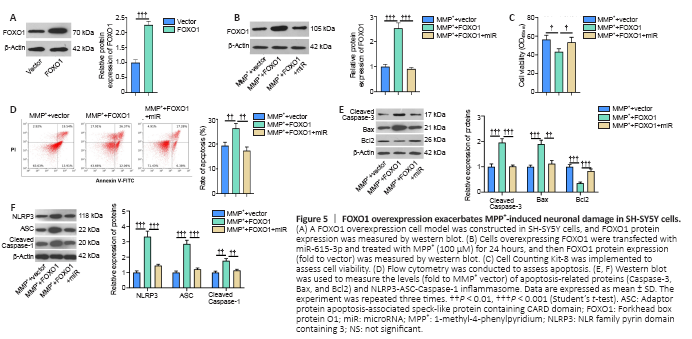
We constructed a FOXO1 overexpression SH-SY5Y cell model to investigate the effect of FOXO1 on neurons in PD (Figure 5A). We transfected miR-615-3p into cells overexpressing FOXO1, and FOXO1 was suppressed in the MPP+ + FOXO1 + miR group compared with that in the MPP+ + FOXO1 group (P < 0.05; Figure 5B). Next, we tested the levels of neuronal viability and apoptosis. Cell viability and Bcl2 expression were suppressed, whereas cell apoptosis and Caspase-3 and Bax expressions were elevated in the MPP+ + FOXO1 group compared with MPP+ + vector group (P < 0.05; Figure 5C–E). In contrast, SH-SY5Y viability was enhanced and apoptosis was reduced in the MPP+ + vector group compared with that of the MPP+ + FOXO1 group (P < 0.05; Figure 5C–E). Subsequently, we measured the expression of NLRP3-ASC-Caspase-1 inflammasomes. FOXO1 overexpression facilitated the expression of NLRP3-ASC-Caspase-1, and this effect was attenuated by miR-615-3p (Figure 5F). Thus, FOXO1 upregulation exacerbated the MPP+-induced neuronal damage, possibly by activating NLRP3-ASC-Caspase-1 inflammasomes.
Figure 6|FOXO1 enhances the role of MPP+-induced inflammation in microglia (BV2 cells).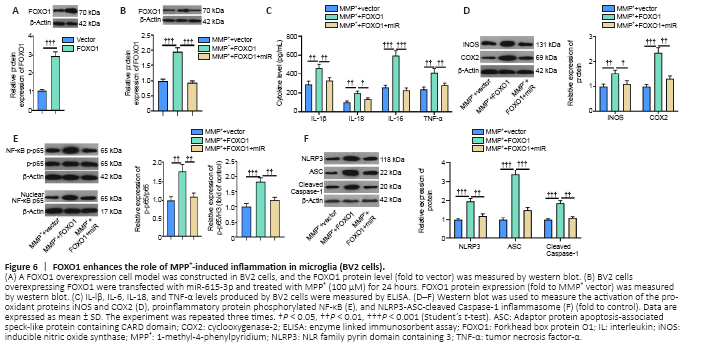
Next, we constructed a FOXO1 overexpression model in BV2 cells (Figure 6A) and showed that miR-615-3p overexpression suppressed FOXO1 expression (Figure 6B). Subsequently, we treated BV2 cells with MPP+ and detected the expression of inflammatory factors and proteins by ELISA and western blot. IL-lβ, IL-6, IL-18, TNF-α, iNOS, COX2, and phosphorylated NF-κB were upregulated in the MPP+ + FOXO1 group compared with the MPP+ group (P < 0.05; Figure 6C–E). In contrast, miR-615-3p overexpression inhibited the above-mentioned inflammatory response (P < 0.05; Figure 6C–E). Finally, western blot was implemented to measure the expression of NLRP3-ASC-cleaved Caspase-1 inflammasomes. FOXO1 upregulation further activated the NLRP3-ASC-Caspase-1 inflammasome, and miR-615-3p overexpression dampened this effect (P < 0.05 vs. MPP+ + FOXO1 group; Figure 6F). These results suggest that FOXO1 exerted a proinflammatory effect on MPP+-induced inflammation.
Figure 7|HOTTIP knockdown alleviated neurological dysfunction and neuronal damage in PD mice.
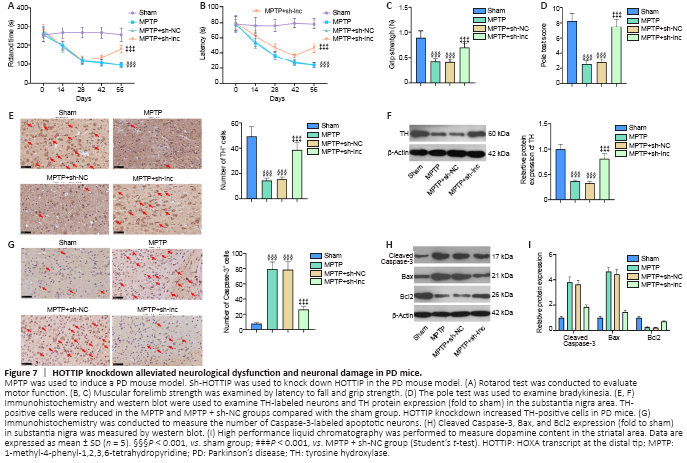
We induced a PD mouse model combined with HOTTIP knockdown to explore the therapeutic effect of HOTTIP in MPTP-induced PD. First, we assessed the neurological changes in mice. Rotarod time and latency to fall were shorter, and grip strength and pole test scores were lower in PD mice than in sham mice (P < 0.001; Figure 7A–D). However, the neurological scores of HOTTIP knockdown PD mice were significantly improved compared with those of control PD mice (P < 0.05; Figure 7A–D).
We also measured the number of TH-labeled neurons and Caspase-3-labeled apoptotic neurons in the SN area. TH-positive cells and TH expression were decreased, whereas Caspase-3-positive cells and Caspase-3 and Bax expressions were increased in PD mice compared with sham mice (Figure 7E–H). Furthermore, HPLC was performed to evaluate dopamine content in the striatal area. Dopamine content was reduced in the MPTP-treated mice compared with sham mice, and HOTTIP knockdown enhanced the dopamine level significantly (Figure 7I). These results suggest that HOTTIP knockdown exerted a neuroprotective effect in PD mice.
Figure 8|HOTTIP knockdown suppresses neuroinflammation and microglial activation in PD mice.
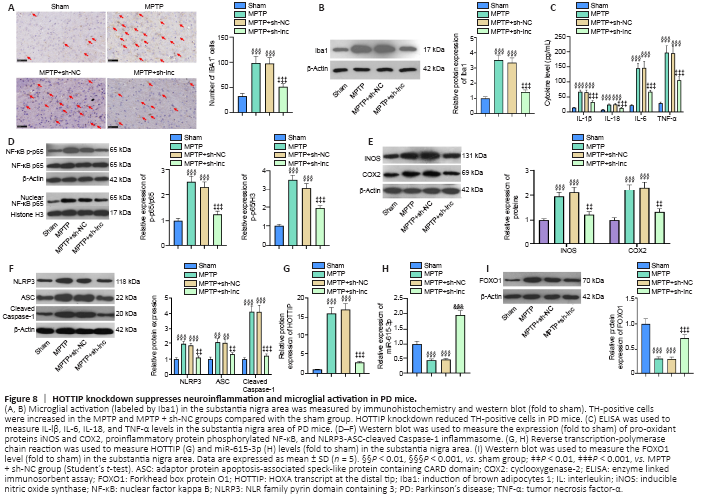
We used immunohistochemistry and western blot to examine microglial activation in the SN area. The number of Iba1-positive cells and Iba1 expression were increased in PD mice compared with sham mice (Figure 8A and B). In contrast, the Iba1-positive cell number and Iba1 expression were both decreased in HOTTIP knockdown PD mice compared with control PD mice (Figure 8A and B).
In addition, the expression of inflammatory cytokines and proteins was measured in the SN area. IL-lβ, IL-6, IL-18, TNF-α, iNOS, COX2, and phosphorylated NF-κB were upregulated in the PD group compared with the sham group, and the increased expression of these factors was ameliorated in the HOTTIP knockdown PD group compared with the MPTP + sh-NC group (P < 0.05; Figure 8C–E). Furthermore, HOTTIP knockdown alleviated MPTP-induced NLRP3-ASC-Caspase-1 inflammasome activation (P < 0.05; Figure 8F). In the SN area, HOTTIP levels were increased, whereas miR-615-3p and FOXO1 levels were decreased in the MPTP group compared with the sham group (P < 0.001; Figure 8G–I). HOTTIP was downregulated, whereas miR-615-3p and FOXO1 were upregulated in HOTTIP knockdown PD mice compared with control PD mice (P < 0.001; Figure 8G–I). The above results suggested that the downregulation of HOTTIP alleviated neuroinflammation in the PD model by regulating miR-615-3p and FOXO1.