周围神经损伤
-
Figure 1|WGCNA and identification of significant DEG modules from proximal nerve segments.
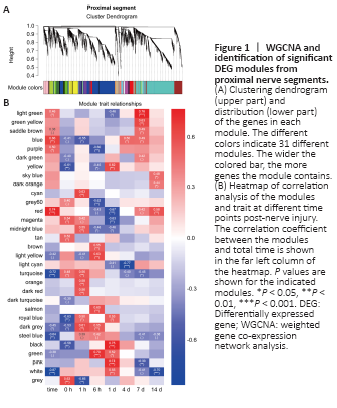
Figure 2|WGCNA and identification of significant DEG modules from distal nerve segments.
To systematically analyze and compare gene expression between proximal and distal nerve segments, we first performed a microarray analysis and found that there were 17,482 and 13,988 differentially expressed genes at 0, 1, and 6 hours, and 1, 4, 7, and 14 days post-sciatic nerve transection in the proximal and distal nerve stumps, respectively. We then used WGCNA, a method for transforming gene expression data into co-expression modules, to separately analyze the differentially expressed genes in the proximal and distal nerve stumps. Before constructing the co-expression modules, we filtered out differentially expressed genes with low expression variation (standard deviation ≤ 0.25), yielding 13,331 proximal and 10,726 distal genes. Power value is another critical parameter that affects the independence and average degree of connectivity of co-expression modules. Hence, we screened a range of power values and found that a power value equal to 14 resulted in high degrees of independence and average connectivity (Additional Figure 1). When this power value was used to generate the weighted co-expression network model, the 13,331 genes identified in the proximal segment were divided into 31 modules (Figure 1A). We next applied a Pearson correlation algorithm to calculate the correlation coefficient and P values of representative module genes and time to determine the importance of each proximal module (Figure 1B). In parallel, the 10,726 genes of distal segment were divided into 15 modules (Figure 2A), and the relative significance of the 15 modules was also determined based on Pearson correlation analysis (Figure 2B). The genes included in each module are listed in Additional Table 2. The significant modules for both the proximal and distal segments are shown in Figures 1B and 2B.
Figure 3|Functional KEGG analysis of gene enrichment in modules that correlate positively with time.

As described above, we used WGCNA to identify significant gene co-expression modules in proximal and distal nerve stumps from rats after sciatic nerve injury. Next, we performed KEGG and GO functional analyses of genes in selected modules to further understand the functional differences and similarities between the proximal and distal modules. There were significant differences in the KEGG pathways and biology processes that were enriched in the proximal and distal modules. The red proximal module was mainly enriched in genes related to axon guidance, extracellular matrix-receptor interaction, cell adhesion, and cyclic adenosine monophosphate signaling (Figure 3A), while the purple and blue proximal modules were mainly enriched in genes related to lysosomes, the cell cycle, DNA replication, and cytokine-cytokine interactions (Figure 3B and C). Most of these pathways have been previously reported to be involved in nerve regeneration. The distal modules were primarily enriched in genes involved in degeneration-related pathways such as calcium signaling, lysosomes, glycan degeneration, and fatty acid degeneration (Figure 3D–F). Consistent with this, GO enrichment analysis showed that the proximal modules were enriched in genes involved to nerve regeneration–related processes, and distal modules were enriched in genes related to nerve degeneration (Additional Figure 2).
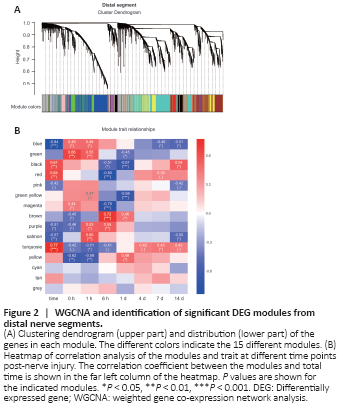
Figure 4|Functional KEGG analysis of genes enriched in modules that correlate negatively with time.
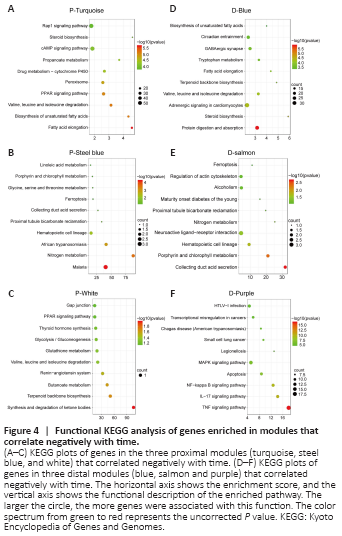
Next, we performed functional enrichment analysis of genes in the proximal and distal modules that were negatively correlated with time (Figure 4 and Additional Figure 4). As shown in Figure 4A–C, the three proximal modules were primarily enriched in genes involved in metabolism-related pathways such as fatty acid elongation, unsaturated fatty acid biosynthesis, and nitrogen metabolism. Axon regeneration after injury requires a large supply of energy, so these functions, as well as those of other metabolic pathways that store or consume large amounts of energy, may be down-regulated to help the damaged neurons successfully regenerate without undergoing an energy crisis. Two distal segment modules were also enriched in genes related to metabolism (Figure 4D and E). The relevant KEGG pathways are listed in Additional Tables 3 and 4. Another distal module, the purple module, was enriched in genes involved in inflammatory- and apoptosis-related signaling pathways, which is consistent with the results from the GO enrichment analysis described above (Figure 4F). As discussed above, although genes in the purple distal module were negatively correlated with time overall, they were up-regulated at 1 and 6 hours post-injury, presumably due to participation in the initiation of WD. Furthermore, several biological processes were enriched in both proximal and distal modules (Additional Figure 5). In brief, processes associated with response to injury such as wound healing and drug response were up-regulated in both proximal and distal segments (Additional Figure 5A), while processes associated with energy-storing metabolic processes such as cholesterol and sterol biosynthesis were down-regulated in both segments over time (Additional Figure 5B). Taken together, our results suggest that peripheral nerve repair is a dynamic process that requires analysis at different time points, and that WGCNA can provide comprehensive insight into this process.
Figure 5|Kinesin family member 22 (Kif22) is the key hub gene that correlates positively with time in both the proximal and distal segments.

To identify key genes regulating axon regeneration and WD, we selected the blue proximal module and the turquoise distal module for further analysis. In total, 544 and 264 critical genes that were positively associated with time were identified in the blue proximal module and the turquoise distal module, respectively, based on an MM threshold of > 0.9 and a GS threshold of > 0.5 (Figure 5A and B and Additional Table 5). Next, Cytoscape software was used to calculate intramodular connectivity within each of the two modules and identify the 50 genes in each module with the greatest degree of intramodular connectivity, which we designated as hub genes (Figure 5C and D). Interestingly, we found that Ngfr and Kif22 were not only critical genes associated with time (Additional Table 5), but were also hub genes in both the blue proximal module and the turquoise distal module (Figure 5C and D). Ngfr, or nerve growth factor receptor, has been previously shown to be up-regulated in SCs after nerve injury, and to interact synergistically with nerve growth factor to guide axon regeneration and activate autophagy in SCs to enhance myelin debris clearance during WD, thus playing important role in peripheral nerve repair (Zhou and Li, 2007; Webber et al., 2008; Jessen and Mirsky, 2016; Li et al., 2020b). Kif22 is a microtubule positive-end tracking protein that is crucial for cell mitosis, centrosome separation, and spindle formation (Yu et al., 2020). Previous studies have shown that Kif22 promotes cancer cell proliferation and metastasis (Yu et al., 2014, 2020). However, the role of Kif22 in peripheral nerve repair is currently unknown. To verify the microarray and WGCNA results for Kif22, we performed quantitative real-time polymerase chain reaction and found that Kif22 mRNA expression was significantly up-regulated from 4 days post-nerve injury in both the proximal and distal nerve stumps (Figures 1A, 5E and 5F), indicating a potential role for Kif22 in axon regeneration and WD. Collectively, these results suggest that Kif22 activity is an important part of peripheral nerve repair.
Figure 6|The effect of Kif22 knockdown on SC proliferation and migration.
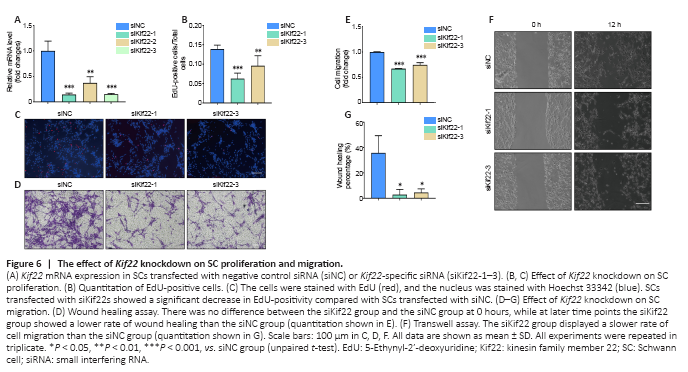
SCs, which are the most important glial cells in the peripheral nervous system, play an essential role in peripheral nerve development and function, and especially in peripheral nerve repair after injury. After injury, myelinating and non-myelinating (Remak) SCs de-differentiate, activate, or reprogram to convert into repair SCs (Jessen and Mirsky, 2016). Then, the repair SCs further proliferate, migrate, form bands of Büngner to guide axon regeneration, and secrete a variety of cytokines and neurotrophic factors to activate macrophages to engulf myelin fragments, thereby initiating WD and promoting nerve regeneration (Jessen and Mirsky, 2016; Jessen and Arthur-Farraj, 2019). Given the role of SCs in axon regeneration and WD and the critical role of Kif22 in regulating cancer cell proliferation and metastasis, we next asked whether the effect of Kif22 on SC proliferation and migration promotes peripheral nerve repair. First, we designed three Kif22-specific siRNAs to knock down Kif22 expression in SCs. As shown in Figure 5A, all three Kif22-specific siRNAs significantly reduced Kif22 expression. We used the two most effective Kif22-specific siRNAs to silence Kif22 in SCs and investigate the effect of Kif22 knockdown on SC proliferation and migration. An EdU assay showed that Kif22 knockdown decreased the rate of SC proliferation (Figure 6B and C), and a Transwell assay showed that Kif22 knockdown disrupted SC migration (Figure 6D and E). The wound healing assay further validated the negative effect of Kif22 knockdown on SC migration (Figure 6F and G). Taken together, these results suggest that Kif22 promotes SC proliferation and migration.
Figure 7|Kif22 knockdown decreases PKCα expression, resulting in inactivation of the ERK signaling pathway.
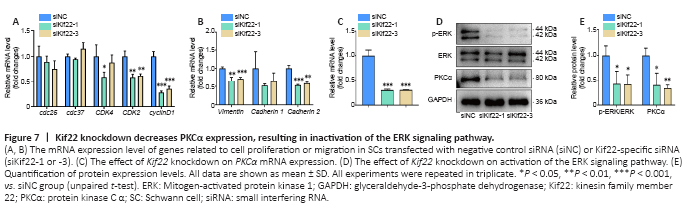
We next explored the mechanisms underlying Kif22-mediated regulation of SC proliferation and migration. We first analyzed the effects of Kif22 knockdown on the expression of genes involved in cell cycle and migration. In general, Kif22 knockdown stably decreased the expression of several genes involved in the cell cycle, including cyclin dependent kinase 2 and cyclin D1 (Figure 7A). Cyclin D1 was identified as a critical gene associated with time in both the blue proximal module and the turquoise distal module based on an MM threshold of > 0.9 and a GS threshold of > 0.5 (Additional Table 5). Consistent with this finding, cyclin D1 has been previously reported to be down-regulated by Kif22 silencing, thereby inhibiting the proliferation of gastric cancer cells (Yu et al., 2020). These findings strongly suggest that Kif22 knockdown can drastically decreased the expression of cyclin dependent kinase 2 and cyclin D1 to disrupt SC proliferation. Kif22 knockdown also significantly reduced the expression of genes involved in cell migration, such as vimentin and cadherin 2 (Figure 7B). These two genes encode key adhesion molecules that regulate SC migration (Clements et al., 2017; Yao et al., 2020). Therefore, our findings suggest that Kif22 knockdown inhibits SC migration by modulating the expression of vimentin and cadherin 2.
Next, we explored the mechanism by which Kif22 contributes to altering the expression of genes related to the cell cycle and migration. The ERK signaling pathway plays an important role in SC proliferation and migration, as well as regulation of axon regeneration and WD (Napoli et al., 2012; Li et al., 2020a). The ERK signaling pathway is regulated by numerous upstream signals, including epidermal growth factor, insulin-like growth factor, and other growth factor signals, as well as PKCα (Yin et al., 2013; Sun et al., 2015). A previous study found that PKCα is up-regulated in rat SCs from distal sciatic nerve stumps after injury and activates the ERK signaling pathway to promote SC proliferation and migration (Li et al., 2020a). However, here we found that PKCα was down-regulated by Kif22 knockdown in SCs (Figure 7C–E). Moreover, a previous study reported that Kif22 is related to the ERK signaling pathway, and that Kif22 knockdown reduces p-ERK expression levels in gastric cancer cells (Yu et al., 2020). Thus, we asked whether altering Kif22 expression would affect activation of the ERK signaling pathway in SCs. Western blot analysis showed that knocking down Kif22 in SCs with Kif22-specific siRNAs significantly inhibited ERK phosphorylation (Figure 7D and E). Taken together, these date indicate that Kif22 modulates SC proliferation and migration by affecting PKCα expression and thereby activating the ERK signaling pathway.