周围神经损伤
-
Figure 1| Experimental design.
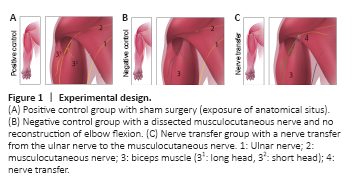
To test surgical feasibility, anatomical dissections were conducted bilaterally in four euthanized Sprague-Dawley neonates (Strain Code 400, Charles River Laboratories, Wilmington, MA, USA) studying the neuromuscular anatomy of the neonatal brachial plexus. To optimize in vivo explorations, preliminary surgical and anesthesiologic tests were conducted in eight pilot neonates. Subsequently, the core experiment was conducted in 25 neonate rats (male, 6–8 g), which were operated within 24 hours after birth (Figure 1). Due to three operative drop outs in the core experiment a total of 28 rats were included in this study. Littermate pups were blindly assigned to the following three groups. For the positive control group, ten animals received sham surgery with skin incision, exposure of the relevant nerves, and closure (sham group). The musculocutaneous nerve (MCN) with its targets was explored in five of these sham animals and the ulnar nerve (UN) in the remaining five. For the negative control group, the MCN was dissected and divided in five animals, but here, no reconstruction was performed. In order to guarantee better comparability, in the negative control group, the MCN was reflected back and fibrin glue was also applied. A total of ten animals underwent a SNT, as described below (nerve transfer group). All animals received humane care in compliance with the principles of laboratory animal care as recommended by Federation of European Laboratory Animal Science Associations (FELASA) (Guillen, 2012). Approval was obtained prior to the study from the ethics committee of the Medical University of Vienna and the Austrian Ministry for Research and Science (BMWF: Bundesministerium fuer Wissenschaft und Forschung, reference number: BMWF-66.009/0187-WF/V/3b/2015, approval date: March 20, 2015).
Figure 2|Surgical anatomy in the neonatal rat.
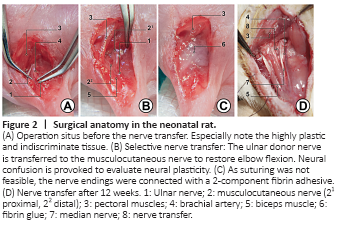
Prior to the core experiment, brachial plexus anatomy was bilaterally explored in four euthanized animals and a selective nerve transfer was found to be surgically feasible. The neonatal brachial plexus and its terminal branches were analogous to human anatomy. However, when operating within 24 hours after birth, not only neural but also muscular and connective tissue were still indiscriminate and of high plasticity (Figure 2). Hence, the procedure and the perioperative management had to be iteratively optimized in eight pilot neonates.
Figure 3|Nerve transfer surgery in the neonatal rat.
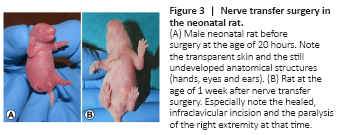
In five out of eight pilot animals the nerve transfer surgery was performed successfully, and the initial operation was survived (drop out = 37.5%). In the core experiment (n = 28) mean operative complication rate decreased from 14% (n = 2 out of 14) in the first half of operated animals to 7% in the second (n = 1 out of 14). The overall drop-out rate was statistically significantly reduced over the course of the experiment (P < 0.001). Summarized, in six animals intraoperative fatal complications were documented. Intraoperative fatal complications resulted from anesthesia difficulties, arterial or venous bleeding. Anesthesia difficulties (n = 3) were accompanied by complete respiratory arrest. Following arterial injury (n = 2) and the corresponding blood loss, death was imminent. One animal died within twelve hours postoperatively due to continuous venous bleeding (n = 1). In the core experiment, mean time between skin incision and closure of the nerve transfer surgeries was 33.5 ± 5.3 minutes. Postoperatively, neonates showed no signs of pain or distress under the given pain medication scheme and wound healing was uneventful (Figure 3).
Figure 4| Regenerative process in the Bertelli test.
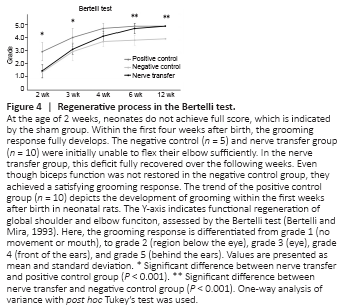
Compared to the contralateral side, an impaired and delayed development of the operated extremity was evident in the nerve transfer group for about 20 days. Here, simple movements were restricted and the development of a stable gait was delayed. These limitations were evident in the negative control group for about 6 weeks and were not found in sham animals. The regenerative process was also apparent in the Bertelli Test. Here animals in the negative control and nerve transfer groups achieved scores of 1.5 ± 0.5 and 1.4 ± 0.5, respectively, at the age of 2 weeks (P = 0.949). This resulted from both initially being unable to flex their elbow sufficiently. Even though the sham group only achieved 3 ± 0.7 out of 5 points at the same age, the difference was statistically significant compared with the transfer animals (P < 0.001; Figure 4). However, this deficit recovered continuously and faster over the following weeks in the nerve transfer group than in the negative control (Supplementary Video). Six weeks postoperatively, there was no statistically significant difference found between the sham (5 ± 0) and nerve transfer group (4.8 ± 0.4; P = 0.382). At this age, the negative control group only achieved 3.8 ± 0.4, which was significantly lower than in the nerve transfer group (P < 0.001). After 12 weeks, nerve transfer and sham animals both were all able to reach behind the ears and, thus, scored the maximum of five points. The negative control animals never achieved this grade of recovery (4 ± 0; Figure 4).
Figure 6| Histological muscle assessment.
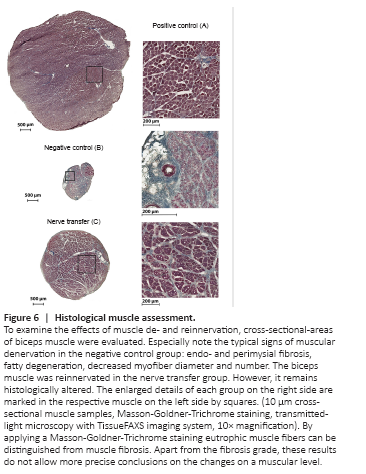
Goldner-Trichrome staining, eutrophic muscle fibers were distinguished from muscle fibrosis. In the negative control, endo- and perimysial fibrosis, along with fatty degeneration, decreased myofiber diameter, and degenerated, atrophic muscle fibers were observed (Figure 6). In this group, the remaining hypotrophic MA measured 0.82 ± 0.28 mm2. In the nerve transfer group, fibrotic processes were partially observed and MA reduced accordingly. MA measured 7.07 ± 1.4 mm2 in the transfer (n = 8), and 28.37 ± 6.62 mm2 in the sham group. The differences found in MA were statistically significant between the nerve transfer and both control groups (P < 0.001 and P < 0.001). The MW totaled 83.7 ± 12.3, 269.0 ± 23.2, and 22.1 ± 5.9 mg in the nerve transfer, sham and negative control groups, respectively. The MW again significantly differed between the nerve transfer and both control groups (P < 0.001 and P < 0.001) (Figure 5 and 6).
Figure 7| Motoneuron analysis of the spinal cord.
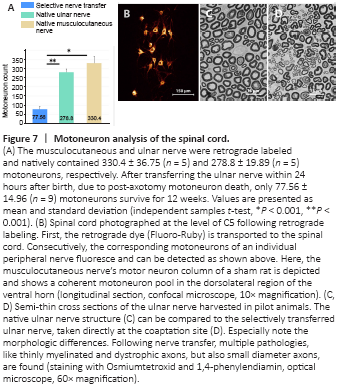
Motoneurons were consistently located in coherent motoneuron pools of the dorsolateral region of the ventral horn. In the nerve transfer group, all motoneurons were identified in the ventral roots of the spinal nerves C8 and Th1. Following the nerve transfer within 24 hours after birth, 77.56 ± 14.96 motoneurons of the UN survived and regenerated for twelve weeks (Figure 7). The native UN was retrogradely labeled and contained 278.8 ± 19.89 motoneurons. This difference was statically significant (P < 0.001) and the ratio of counted motoneurons corresponded to a 74% decline. Natively, the MCN contained 330.4 ± 36.75 motoneurons, inter alia originally innervating the evaluated biceps muscle. Compared with the nerve transfer group, a statically significant difference (P < 0.001) was found (Figure 7).