脊髓损伤
-
Figure 1|Open field locomotor recovery as assessed with BBB scoring.
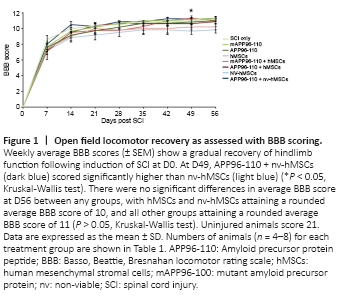
After the complete loss of HL function immediately following SCI (D0) there was a gradual recovery of function over the 8 week experimental period (Figure 1). However, while the nv-hMSC group consistently showed the lowest BBB scores, statistical analysis did not reveal any significant differences in the functional recovery between SCI only and any treatment group, nor between groups, for any functional test up to D56. No group recovered to pre-injury scores. There was a significant increase in BBB score with APP96-110 + nv-hMSCs compared to nv-hMSCs at D49 (P < 0.05; Figure 1), however this was not maintained for the following (final) week. By D56, all groups demonstrated occasional to frequent weight-supported plantar stepping (BBB 10 to 11) with no significant differences in the average BBB score between any groups at D56 (Figure 1; P > 0.05).
Figure 3|Immunostaining of adjacent longitudinal sections of the same injured spinal cord grafted with donor hMSCs, examined at D56 (49 days post-transplantation) revealing neuronal marker (βIII tubulin+, GAP43+ and SMI32+ fiber) profiles in injury sites.
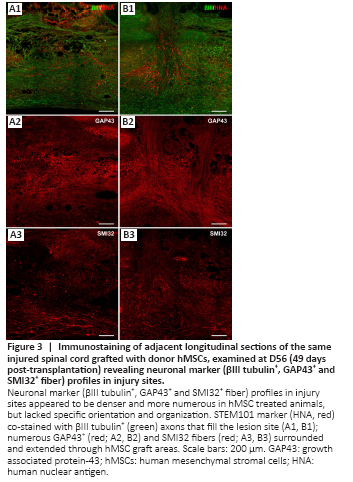
Areas with grafted hMSCs appeared in toluidine blue stained sections as dark blue stained, dense, fibrous areas that had a particular alignment within the tissue (extending rostral/caudal from the injection site or dorsal/ventral along the transplantation needle track – data not shown). Neuronal marker profiling identified a clear injury site that was void of organized neuronal tissue, lacked distinguishable grey and white matter regions and was bordered rostrally and caudally by uninjured tissue, and ventrally by spared tissue. In some cases, the injury site extended up to 6 mm in length (rostral/caudal). The number of βIII tubulin+, GAP43+ and SMI32+ fibers varied greatly between groups and appeared to be denser and more numerous in hMSC treated animals. The majority of neuronal fibers extending within the lesion site lacked specific orientation and organization, however many fibers were orientated with the hMSC graft in hMSC treated animals. At D56 (49 days post-transplantation) numerous βIII tubulin+ axons filled the lesion site and were closely associated with STEM101+ hMSCs (Figure 3A1 and B1). Similarly, numerous GAP43+ (Figure 3A2 and B2) and SMI32+ (Figure 3A3 and B3) fibers surrounded and extended through hMSC graft areas. Neuronal fibers appeared to be denser and more aligned when associated with hMSC graft areas, perhaps indicating that the grafted cells promoted and/or supported the regrowth/regeneration of neuronal processes.
Figure 4|Immunostaining of adjacent longitudinal sections of the same injured spinal cord grafted with donor hMSCs examined at D56 (49 days post-transplantation) revealing astrocytic (GFAP+), extracellular matrix (laminin+) and microglial/macrophage (ED1+) marker profiles in injury sites.
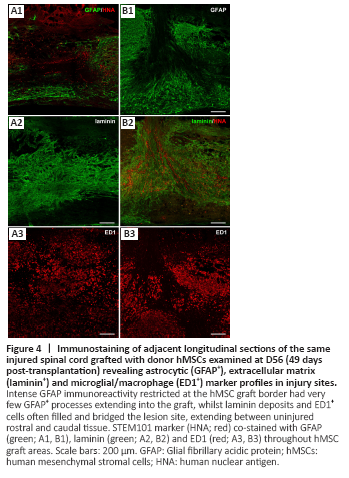
Astrocytes (S100+ or GFAP+) were evenly distributed throughout uninjured tissue and present within the lesion site to varying degrees. The lesion site was largely devoid of S100 and GFAP immunoreactivity, particularly in cell transplanted animals. GFAP immunoreactivity was strongest in tissue immediately adjacent to the injury site and/or hMSC graft regions. There was limited interaction between donor hMSCs and host glial cells, identified by GFAP (astrocytes) or S100 (astrocytes and perhaps infiltrating Schwann cells) immunoreactivity. As shown in Figure 4A1 and B1, there was intense GFAP immunoreactivity at the border between host tissue and the hMSC graft with very few GFAP+ processed extending into the graft. S100 immunoreactivity followed a similar pattern with abundant S100+ profiles surrounding the graft and few S100+ process extending into the graft area (not shown).
Laminin deposits filled and bridged the lesion site of all animals, extending between uninjured rostral and caudal tissue. Extensive tubular laminin deposits filled the lesion site of all hMSC transplanted animals and appeared to be greatest in areas within and immediately surrounding the hMSC graft (not quantified; Figure 4A2 and B2). Accordingly, laminin+ profiles were often orientated with the direction of the hMSC graft.
Iba1+ and ED1+ microglia/macrophages were present within the lesion site and in surrounding uninjured tissue of all animals at 8 weeks post-SCI. Iba1 and ED1 immunoreactivity identified resident and infiltrating microglia/macrophages within the injury site that had either an amoeboid morphology with short processes characteristic of activated microglia or a large, foamy/spongy morphology characteristic of phagocytotic microglia/macrophages (ED1+, Figure 4A3 and B3). Iba1+ cells present within the injury site often aligned with the hMSC graft while ED1+ cells were often concentrated at the border of the graft with few ED1+ cells infiltrating the graft area. ED1 immunoreactivity appeared weaker within graft areas than the surrounding tissue, suggesting that donor hMSCs may be influencing the activation and infiltration of microglia/macrophages to varying degrees.