脑损伤
-
Figure 1 | A schematic diagram of formed ischemic core and ischemic penumbra regions.
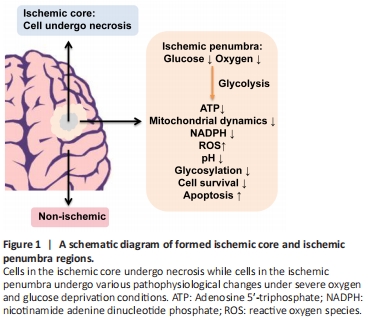
Stroke is one of the leading causes of morbidity and mortality worldwide with ischemic stroke accounting for more than 80% of the cases (Feigin et al., 2017). Ischemic stroke is caused by obstruction of cerebral arteries, leading to a lack of regional cerebral blood supply causing severe oxygen and glucose deprivation (OGD), and irreversible neuronal damage and death in the brain tissue (Figure 1) (Robbins and Swanson, 2014; Puig et al., 2018). To develop effective therapeutics for ischemic stroke, tremendous efforts have been made to reveal its pathogenic mechanisms and test various treatments (Puig et al., 2018; Huang and Zhang, 2019; Barthels and Das, 2020). Currently, mechanical thrombectomy or in combination with thrombolytic agents is major effective treatment, which has significantly improved clinical outcomes. However, no further treatment has been made from bench to bedside since tissue plasminogen activator was approved by the Food and Drug Administration for acute ischemic stroke in 1996. Moreover, less than 5% of stroke patients are eligible for tissue plasminogen activator therapy due to its narrow therapeutic window with multiple contraindications (Barthels and Das, 2020) while exploiting neuroprotectants for treatment persists a challenge (Muresanu et al., 2019). Therefore, it is essential to further understand cerebral ischemia-induced pathophysiological changes and develop novel therapeutic strategies for ischemic stroke care.
Figure 2 | Possible benefits from low glucose supply via blood glucose management or onsite low glucose supply.
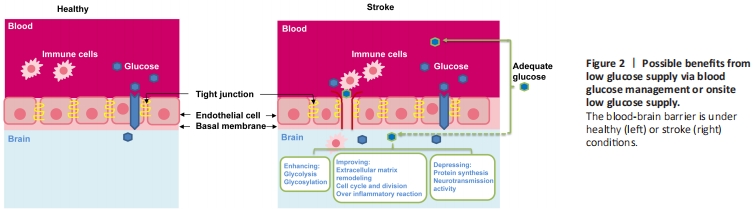
Both glucose entry from the blood to the brain and its metabolism in the brain under normal physiological conditions are tightly regulated (Mergenthaler et al., 2013; Zhang et al., 2014; Koepsell, 2020). The blood-brain barrier (BBB) and glucose transporters (GLUTs) play critical roles in controlling glucose transport to the brain (Figure 2). The BBB selectively controls inward and outward transportation of glucose, oxygen, and many other substances through cell junctions and cell surface transport systems and several GLUTs. Among 15 identified GLUTs, GLUT1 and GLUT3 are considered to be the primary transporters for glucose uptake in the brain (Koepsell, 2020). GLUT1 expression form with a molecular weight of 55-kDa has been confirmed to be highly enriched in the microvascular endothelial cells of the BBB while GLUT3 is predominantly expressed in neurons with a higher transport rate to facilitate neuronal glucose uptake (Zhang et al., 2014; Koepsell, 2020). In addition to glucose transport, some cerebral glucose transporters also play a critical role in sensing glucose concentrations in the blood and brain. GLUT2 and GLUT4 are expressed in neurons and astrocytes where they also act as glucose sensors and are involved in the regulation of glucose homeostasis. Furthermore, GLUT5, GLUT6, and GLUT8 were found to be expressed in various regions of the brain, but the physiological roles of these as well as the remaining other members in the brain are still unclear (Zhang et al. 2014; Koepsell, 2020).
Figure 3 | Utilization of UDP-GlcNAc synthesized from glucose in protein glycosylation.
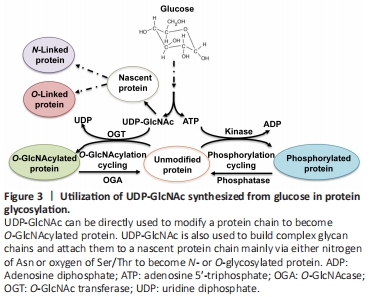
Cerebral glucose also plays important regulatory roles in glycosylation (Figure 3) (Videira et al., 2018; Paprocka et al., 2021).