脑损伤
-
Figure 1|Rapid and efficient astrocyte-to-neuron conversion in the ischemic cortex.
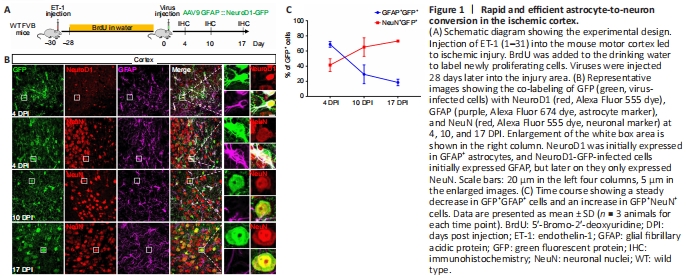
Our group and other groups have demonstrated that reactive astrocytes can be directly converted into neurons by ectopically expressing neural transcription factors or knocking down PTBP1, leading to tissue and function repair of the injured brain (Rivetti di Val Cervo et al., 2017; Chen et al., 2020; Qian et al., 2020; Wu et al., 2020). However, this in vivo AtN conversion has been challenged recently owing to confusion largely caused by a high dose of AAV used in some studies that inevitably led to a so called “leakage” of the transgene signal directly into neurons near the targeted glial cells (Wang et al., 2020). Another concern was raised on the basis of a BrdU incorporation experiment in which the number of BrdU-labeled neurons was very small after in vivo AtN conversion (Wang et al., 2020). To investigate why few BrdU-labeled neurons remained after conversion, we established ET-1-induced ischemic injury in the FVB mouse motor cortex and continuously administrated BrdU in the drinking water to label dividing glial cells (Figure 1A). At 30 days after the injury (continuous 28-day BrdU administration), AAV9 GFAP::NeuroD1-P2A-GFP was injected into the injured cortical region and animals were sacrificed for analysis at 4, 10, and 17 days after virus infection (DPI) (Figure 1A). Similar to our previous study (Chen et al., 2020), NeuroD1 expression was detected in astrocytes at 4 DPI (Figure 1B, top row) and the NeuroD1-GFP-infected cells gradually changed from GFAP+ astrocytes to NeuN+ neurons from 4 to 17 DPI (Figure 1B). Quantitation showed that the NeuN signal in the infected cells increased from 41% at 4 DPI to 73% at 17 DPI, while the GFAP signal decreased concomitantly from 69% at 4 DPI to 19% at 17 DPI (Figure 1C). These results suggest efficient AtN conversion occurred in the ET-1-induced chronic ischemic injury model in the mouse motor cortex.
Figure 2|Decrease in BrdU-positive cells during astrocyte-to-neuron conversion.

Analysis of the number of BrdU-labeled cells during the conversion revealed that the proportion of both BrdU-positive astrocytes and the total BrdU-positive cells among the NeuroD1-GFP-infected cells gradually decreased (Figure 2A). Namely, the ratio of BrdU-positive astrocytes among the NeuroD1-GFP-infected cells declined from 32% at 4 DPI to 13% at 17 DPI (P = 0.0012 for 4 DPI vs. 10 DPI, P = 0.0006 for 4 DPI vs. 17 DPI; Figure 2B and C). Similarly, among the GFAP+ astrocytes, the ratio of BrdU-positive astrocytes also decreased from 75% at 4 DPI to 49% at 17 DPI (P = 0.015; Figure 2D). This phenomenon indicated a gradual loss of BrdU-labeled cells during NeuroD1-induced AtN conversion. We then investigated whether this reduction is specifically related to NeuroD1 by infecting the astrocytes with a control AAV GFAP::GFP. Surprisingly, the proportion of BrdU-labeled astrocytes did not show a significant change among the GFP-infected cells, suggesting that BrdU did not affect normal dividing glial cells (Figure 2E–G). Therefore, the reduction in BrdU-positive cells in the NeuroD1 group is potentially linked to the AtN conversion.
Figure 3|Few converted neurons are BrdU-positive.
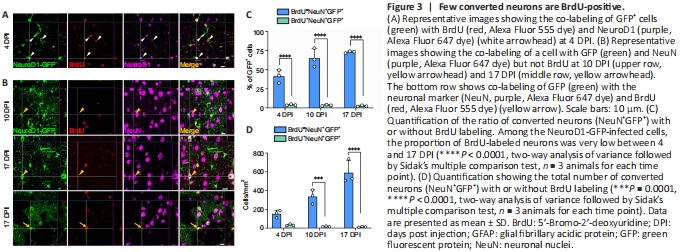
Because the ratio of BrdU-labeled astrocytes decreased among the NeuroD1-GFP-infected cells, we wondered whether this portion of astrocytes can transdifferentiate into neurons. To answer this question, we examined the BrdU-positive neurons within the NeuroD1-converted neurons at different stages of AtN conversion. At 4 DPI, the expression of NeuroD1 was clearly observed in BrdU-positive astrocytes (Figure 3A), suggesting that incorporation of BrdU into the astrocytes did not affect NeuroD1 expression. As the neuronal reprogramming proceeded, we detected both BrdU-positive and negative neurons (NeuN+) among the NeuroD1-GFP-infected cells (Figure 3B), but the ratio was dramatically different. Strikingly, at the indicated time points during AtN conversion, BrdU-positive neurons only constituted a very small portion of the total NeuroD1-converted neurons (Figure 3C and D). Namely, most of the converted neurons were BrdU-negative, raising the question whether BrdU had any negative effect on the neuronal conversion process.
Figure 4|Efficient BrdU labeling of primary rat astrocytes in vitro.

To investigate whether BrdU affects NeuroD1-induced AtN conversion and avoid potential complications induced by ET-1 ischemic injury, we performed in vitro neuronal conversion experiments using cultured astrocytes in the presence or absence of BrdU. Previous studies have demonstrated that 10 μM BrdU was not toxic to astrocytes and could label ~70% of astrocytes after a short incubation time (Caldwell et al., 2005; Lehner et al., 2011; Schneider and d’Adda di Fagagna, 2012; Zhang et al., 2015). Thus, we performed a 24-hour incubation of 10 μM BrdU with primary cultured rat astrocytes (Figure 4A). The results showed that ~70% of astrocytes were labeled with BrdU after the treatment (Figure 4B and C), suggesting a robust proliferation of the primary rat astrocytes within 24 hours. When cultured rat astrocytes were infected with control retrovirus CAG::GFP, most infected cells were astrocytes and no TuJ1+ neurons were observed by 14 DPI (Figure 4D), suggesting no contaminated neural stem cells within the primary rat astrocyte culture. When retrovirus CAG::NeuroD1-IRES-GFP was used on the primary rat astrocytes without or with BrdU treatment, we observed a sharp contrast between the two groups (Figure 4E). In the group without BrdU treatment, most of the infected astrocytes expressed NeuroD1 and were successfully converted into neuron-like cells at 7 DPI (Figure 4E, top row). However, in the group with BrdU treatment, a significant portion of the infected cells were astrocytes, with relatively strong GFAP expression and weak NeuroD1 expression (Figure 4E and F, bottom row, NeuroD1 intensity in the infected cells at 7 DPI: P = 0.01).
Figure 5|BrdU impaires astrocyte-to-neuron conversion in vitro.

We next examined the effect of BrdU on AtN conversion by comparing the NeuN signal between BrdU-treated and untreated astrocytes at 7 and 14 DPI following NeuroD1-GFP retroviral infection (Figure 5). In the control group without BrdU treatment, most of the NeuroD1-GFP-infected cells showed a NeuN signal at 7 DPI (Figure 5A, top row). However, in the BrdU-treated group, the NeuN signal was very weak among the NeuroD1-GFP-infected cells at 7 DPI (Figure 5A, bottom row). Quantitative analysis showed that without BrdU treatment, NeuroD1 rapidly converted astrocytes into NeuN+ neurons at the high conversion rate of 85% at 7 DPI (Figure 5B). However, in the BrdU-treatment group, the AtN conversion rate dramatically dropped to below 40% (P < 0.0001; Figure 5B). Interestingly, in the BrdU-treatment group, a small portion of astrocytes were not labeled with BrdU (around ~30%), and the AtN conversion rate of these cells (BrdU negative, 34%) was higher than that of the BrdU-positive cells (16%, P = 0.0002). At the later stage of AtN conversion (14 DPI), although the conversion rate increased in both groups, the conversion rate in the BrdU-treatment group remained much lower than that of the non-BrdU group (Figure 5C and D). In addition to the conversion rate decrease induced by BrdU treatment, the number of converted neurons in the BrdU-treatment group was significantly lower than that in the group without BrdU treatment at both 7 and 14 DPI (Figure 5E and F).
Figure 6| BrdU exacerbates cell death during astrocyte-to-neuron conversion.
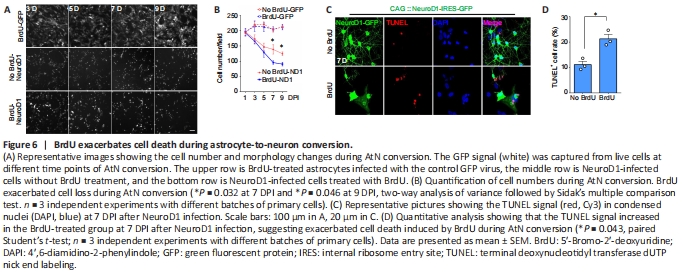
Previous studies have reported toxic effects of BrdU on neural stem cells and neuronal differentiation (Caldwell et al., 2005; Ross et al., 2008; Duque and Rakic, 2011; Lehner et al., 2011; Schneider and d’Adda di Fagagna, 2012). Our findings suggest that BrdU has similar toxic effects on AtN conversion. To further analyze the BrdU effect, we investigated the cell number change during AtN conversion. In the control group where astrocytes were infected with GFP alone, no significant difference “existed between” the BrdU-treated and untreated astrocytes (Figure 6A and B). However, when the astrocytes were infected with the NeuroD1 virus, a dramatic cell morphology switch from astrocyte-like to neuron-like morphology was observed together with a steady cell number decrease, suggesting that some cells did not survive “during” this drastic cell conversion change (Figure 6A and B). Interestingly, the BrdU-treated group showed an even higher cell loss compared with that in the untreated group (Figure 6B; 7 DPI: P = 0.032; 9 DPI: P = 0.046). Next, we performed TUNEL staining to examine whether this cell loss during AtN conversion was partly due to apoptosis as previously reported (Gascón et al., 2016). A TUNEL-positive signal was observed in both the BrdU-treated and untreated groups at 7 DPI after NeuroD1 transduction. However, the TUNEL-positive signal in the BrdU-treated group was significantly higher than that in the untreated group (P = 0.043, paired Student’s t-test; Figure 6C and D).