脑损伤
-
Figure 1|Time course of BDNF expression in the cortical ischemic penumbra and experimental protocol.
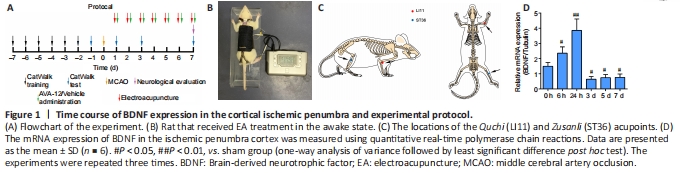
Figure 1A contains a flowchart of the experiment. Rats were anesthetized with an intraperitoneal injection of 30 mg/kg pentobarbital sodium (Shanghai Boyun Biotech, Co., Ltd., Shanghai, China). After making a midline incision at the neck, the left common carotid artery, external carotid artery, and internal carotid artery were exposed. Then, the left internal carotid artery and the common carotid artery distal to the bifurcation were temporarily clamped using microvascular clips. MCAO was performed by gently advancing a nylon monofilament thread with a silicon-coated tip (Jia Ling Biotechnology Co. Ltd., Guangzhou, China) to the origin of the left middle cerebral artery. After 2 hours of MCAO, the monofilament nylon suture was withdrawn to enable the reperfusion process. Rats in the sham group underwent identical surgery, but nylon sutures were not inserted. When a rat rotated to the right while locomoting after MCAO/R surgery, the model was judged to be successful (Liu et al., 2017).
In the MCAO/R + EA + vehicle and MCAO/R + EA + ANA-12 groups, the ST36 and LI11 acupoints were subjected to EA stimulation at the right paralyzed limb once a day between 9:00 a.m. and 11:00 a.m. for 7 days, starting 24 hours after MCAO/R surgery. The rats received EA treatment in the awake state. Specifically, two stainless steel acupuncture needles were inserted into the LI11 (located in the depression lateral to the anterior aspect of the radius joint of the forelimb) and ST36 acupoints (located 5 mm beneath the capitulum fibulae and lateral posterior to the knee joint) (Liu et al., 2016) of the hemiplegic limb at a depth of 2–3 mm (Figure 1B and C). A HANS-200E stimulator (Nanjing Jisheng Medical Co., Ltd., Nanjing, China) was used to deliver dilatational wave stimulations at 2/15 Hz. The stimulus intensity was determined as the point at which slight muscle jitters of the limb could be observed, and the treatment lasted for 30 minutes.
To assess whether the animal model was successfully established, we first examined the time course of BDNF expression in the rat ischemic penumbra. In our experiment, the levels of BDNF increased early after MCAO/R and peaked at 24 hours, which confirmed that MCAO/R caused injury, followed by a slow decline toward the 7th day. Compared with that at hour 0, BDNF expression was lower in the regions containing the ischemic penumbra 7?days after MCAO/R (Figure 1D).
Figure 2| EA improves functional recovery after ischemic stroke in terms of gait.
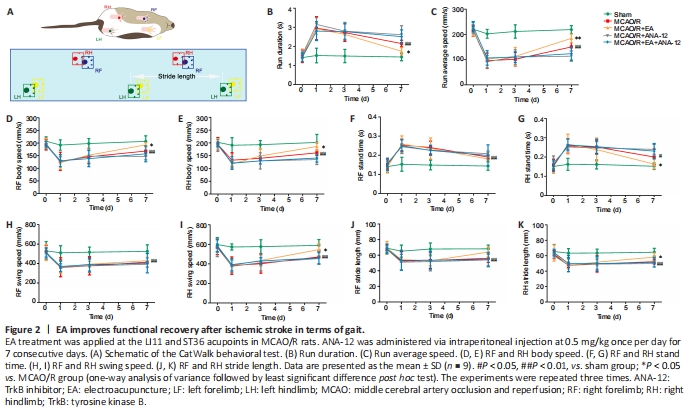
To investigate the effect of EA treatment on motor function in rats after MCAO/R, we conducted gait analyses and compared the MCAO/R + EA and MCAO/R groups. Figure 2A contains a schematic diagram of the CatWalk behavioral test. After the model had been established, the run duration was prolonged (P < 0.01) and the run average speed had decreased (P < 0.01). After EA treatment, the run duration decreased (P < 0.05), indicating an increase in the run average speed (P < 0.01; Figure 2B and C). EA also improved the body speed (P < 0.05; Figure 2D and E). The stand time of the right limbs was severely affected in animals subjected to surgery, and there was no significant difference in stand time for the right forelimb between the MCAO/R and MCAO/R + EA groups (Figure 2F). EA treatment contributed only to the recovery of function in the right hindlimb (P < 0.05; Figure 2G). The swing speed results were in agreement with the stand time results (P < 0.05; Figure 2H and I). Stride length was significantly decreased in the MCAO/R group relative to the sham group (P < 0.01). Nevertheless, the stride length of the right limbs had significantly increased after 7 days of EA administration (P < 0.05; Figure 2J and K).
Figure 3| Effects of EA on MCAO/R-induced brain injury in rats at 7 days after MCAO/R.

We used neurological deficit scores in the present study to examine the neuroprotective effect of EA treatment. In the sham group, no rats displayed symptoms of neurological deficits, whereas there were outstanding neurological deficits in the MCAO/R group. EA treatments significantly reduced the occurrence of neurological deficits in the MCAO/R + EA compared with the MCAO/R group (P < 0.05). Furthermore, intervention with ANA-12 clearly weakened the effect of EA treatments compared with the MCAO/R + EA group (P < 0.01; Figure 3A).
TTC staining revealed no infarctions in the sham group. The sizes of the infarct regions were different in each MCAO/R group. Cerebral infarction was detected in the MCAO/R group, and the infarct volume was significantly increased compared with that in the sham group (P < 0.01). Following the EA intervention, the area of the cerebral infarction significantly decreased compared with the MCAO/R group (P < 0.05). Furthermore, ANA-12 attenuated the effect of EA treatments (P < 0.01; Figure 3B and C). H&E staining indicated that there were no obvious pathological changes in the ischemic penumbra of the sham group; the structure of the tissue was normal, the cells were arranged in an organized manner, and the intercellular space was dense with no edema. Different degrees of vacuolar necrosis, enlarged interspaces, interstitial edema, and shrinking cell nuclei with deep staining were observed in the MCAO/R group (Figure 3D). Additionally, as shown in Figure 3E, the results of Nissl staining demonstrated that the number of pyknotic and fragmented neurons had increased in the MCAO/R group, and the Nissl bodies exhibited signs of dissolution and necrosis. Some deformed and necrotic cells were present in the EA group. The condition of the tissue in the MCAO/R + ANA-12 group was worse than that in the other groups.
Figure 4| EA increases the expression of BDNF and p-TrkB in the cortical ischemic penumbra 7 days after MCAO/R.
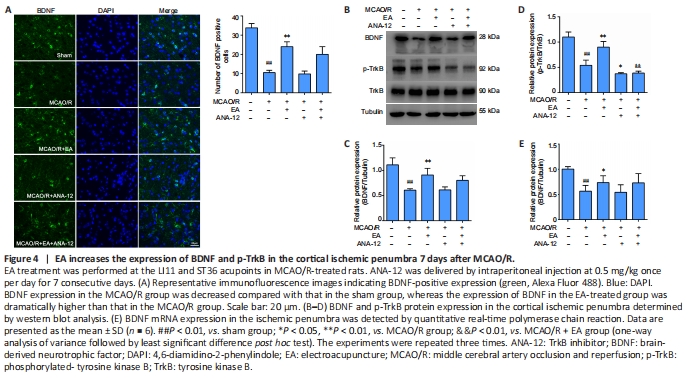
In the present study, we investigated whether the administration of EA affected the expression of BDNF, TrkB, and p-TrkB in the ischemic penumbra after MCAO/R. As shown in Figure 4A, immunofluorescence staining indicated a clear decrease in BDNF immunopositivity in the MCAO/R group compared with the sham group (P < 0.01), whereas there was a marked increase in BDNF levels in the EA group compared with the MCAO/R group (P < 0.01). Western blot analysis revealed a significant reduction in BDNF and p-TrkB expression after modeling compared with the sham group (P < 0.01). Although the administration of ANA-12 did not modify the expression levels of BDNF compared with the MCAO/R group (P > 0.05), the neuroprotective effect of EA on proteins downstream from BDNF was significantly blocked by ANA-12. The protein level of p-TrkB was greatly increased by EA compared with the MCAO/R group (P < 0.01), but was notably decreased by ANA-12 administration compared with the MCAO/R + EA group (P < 0.01). There was no significant difference in TrkB expression between the groups (P > 0.05; Figure 4B–D). The qPCR results revealed an obvious increase in BDNF mRNA levels after EA treatment compared with the MCAO/R group (P < 0.05; Figure 4E).
Figure 5| EA promotes the repair of myelin in the ischemic penumbra and inhibits the expression of Nogo-A and NgR 7 days after MCAO/R.
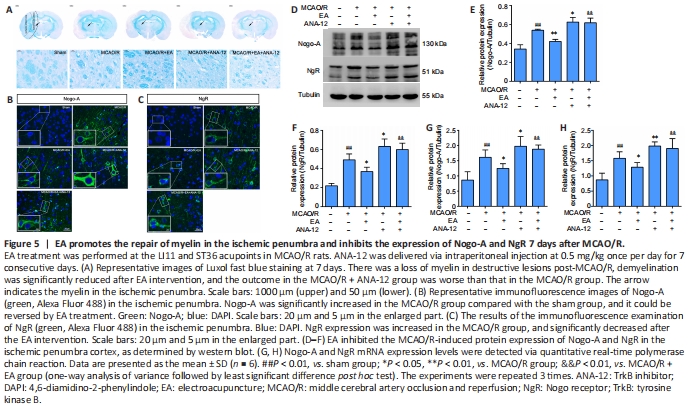
To evaluate the beneficial effect of EA on myelin repair, we performed specific staining using LFB, which is used as a highly sensitive marker of myelin loss, to determine overall myelination (Qin et al., 2018). Although LFB staining showed no evidence of demyelination in the sham group, there was a loss of myelin in destructive lesions post-MCAO/R, and the outcome in the MCAO/R + ANA-12 group was worse than that in the MCAO/R group. Demyelination was significantly reduced in EA-treated rats compared with the MCAO/R group (Figure 5A). Next, we evaluated the expression of Nogo-A and NgR in each group. The immunofluorescence staining suggested that MCAO/R increased the positive expression of Nogo-A and NgR in the ischemic penumbra compared with the sham group (Figure 5B and C). The protein level of Nogo-A in brain tissue was significantly increased in the MCAO/R group compared with the sham group (P < 0.01), and the changes in expression could be reversed by EA treatment (P < 0.01). NgR protein levels in the EA group followed the same trend as the Nogo-A levels (P < 0.05; Figure 5D–F). The same tendency was observed for the mRNA expression levels of Nogo-A and NgR, as determined by qPCR analysis (Figure 5G and H).
Figure 6| EA promotes dendritic branching and growth in the ischemic penumbra.

Figure 6A–C shows representative coronal Golgi-Cox staining illustrating the phenomenon of MCAO/R injury, and the demarcation between the apical and basal dendrites is illustrated in Figure 6D. To investigate changes in dendritic complexity and the mechanism by which EA promotes dendritic growth, we performed Sholl analysis in which we counted the number of intersections along the dendritic trees at all distances from the soma in terms of concentric circles separated by 20 μm (Figure 6E and F). Sholl analysis revealed a significant reduction in the number of intersections along the apical and basal dendrites after ischemia/reperfusion injury compared with the sham group (P < 0.01) (Figure 6G and H). In addition, we found marked and highly significant reductions in dendrite branches in both apical (P < 0.05) and basal (P < 0.01) regions in the MCAO/R group compared with the sham group (Figure 6I and J), with notable reductions in the total lengths of branches (apical branches: P < 0.01; basal branches P < 0.05; Figure 6K and L). Nevertheless, EA effectively rescued the damage to dendrite structure caused by MCAO/R and facilitated regrowth after injury. The dendritic growth was poorest in the MCAO/R + ANA-12 group.
Figure 7| EA reverses impaired spine density and increased levels of synaptic-related proteins in the ischemic penumbra.
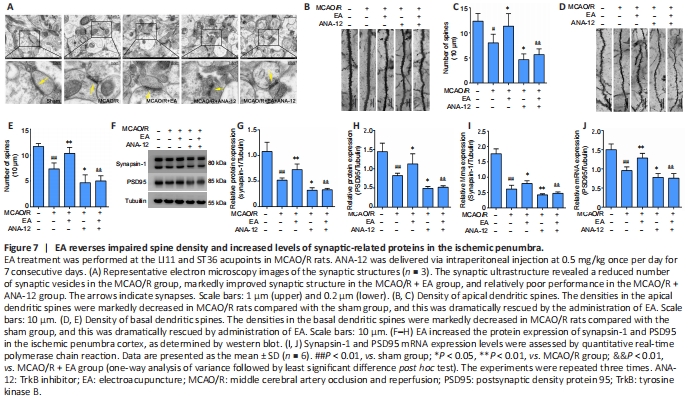
Figure 8| EA promotes the expression of MAP-2 and reduces injury-induced neuronal apoptosis in the ischemic penumbra area.
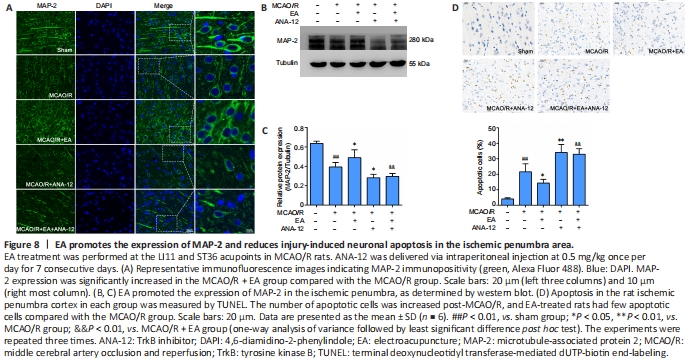
We observed the ultrastructure of the synapses using an electron microscope. Transmission electron microscopy showed that the synapses in the sham group were well preserved, and examination of the synaptic ultrastructure of ischemic penumbra neurons revealed a reduced number of synaptic vesicles after surgery. The synaptic structure in the MCAO/R + EA group was markedly improved after treatment, with thickened synaptic membranes and more synaptic vesicles, as well as tighter synaptic connections. The MCAO/R + ANA-12 group had the poorest performance among the groups (Figure 7A). The generation, maturation, and long-term stabilization of dendritic spines contribute to experience-dependent synaptic plasticity (Padmanabhan et al., 2019). We next determined whether EA affected the plasticity of dendritic spines. The results showed that the densities in both the apical (P < 0.05) and basal (P < 0.01) dendritic spines were markedly decreased in MCAO/R rats compared with the sham group, and that this was dramatically rescued by administration of EA (apical dendritic spines: P < 0.05, basal dendritic spines: P < 0.01; Figure 7B–E). We then investigated the role of EA treatment in synaptic plasticity by evaluating changes in synaptic proteins. EA treatment followed by MCAO/R resulted in significant increases in both the mRNA and protein levels of PSD95 (mRNA: P < 0.01; protein: P < 0.05) and synapsin-1 (mRNA: P < 0.05; protein: P < 0.01) compared with the MCAO/R group (P < 0.05 or P < 0.01; Figure 7F–J), as well as levels of MAP-2 (P < 0.05; Figure 8A–C). We used a TUNEL assay to examine whether the protective effect of EA was associated with a reduction in apoptosis. The results indicated that the number of apoptotic cells had increased post-MCAO/R, and that EA-treated rats had few apoptotic cells compared with the MCAO/R group (P < 0.05). ANA-12 significantly increased the number of apoptotic cells compared with the MCAO/R + EA group (P < 0.01; Figure 8D).
点击此处查看全文