脑损伤
-
Figure 1|Study design.
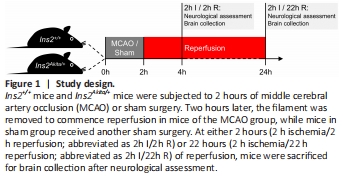
Transient focal cerebral ischemia was induced using the intraluminal method (Lo et al., 2005; Li et al., 2012; Yang et al., 2012). In brief, mice were randomly assigned and were subjected to isoflurane (IsoFlo; 50019100, Zoetis Inc., Kalamazoo, MI, USA) inhalation anesthesia (2% isoflurane in 70% N2O/30% O2 for induction; 1.25% isoflurane in 70% N2O/30% O2 for maintenance). A filament coated with vinylpolysiloxane material (ESPE 7302, 3M Dental Products, St. Paul, MN, USA) was inserted into the right internal carotid artery and further advanced until reaching the middle cerebral artery. After 2 hours of ischemia, the filament was removed to commence reperfusion. Animals in the sham groups received the same procedures except for filament insertion. Relative cerebral blood flow in the middle cerebral artery territory was monitored by a laser Doppler flowmeter (Periflux 5000, Perimed AB, J?rf?lla, Sweden), with the reading at 5 minutes before ischemia set as 100%. Body temperature was maintained at 37 ± 0.5°C throughout the surgery. Mice were placed in an intensive care unit set at 30 ± 0.5°C during ischemia and for 4 hours after reperfusion commenced. Mice were sacrificed at either 2 hours (2 hours ischemia/2 hours reperfusion) or 22 hours (2 hours ischemia/22 hours reperfusion) of reperfusion (Figure 1).
Figure 2| Ins2Akita/+ mice displayed hyperglycemia and decreased body weight.
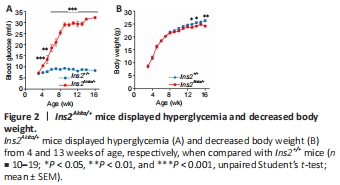
Ins2Akita/+ mice displayed hyperglycemia from 4 weeks of age and a decreased body weight from 13 weeks of age (Figure 2). Ins2Akita/+ mice had higher relative cerebral blood flow during ischemia, which was similar in both genotypes during reperfusion (Table 2).
Figure 3| More severe neurological deficits and lower survival rate in Ins2Akita/+ mice after 2 hours of MCAO.
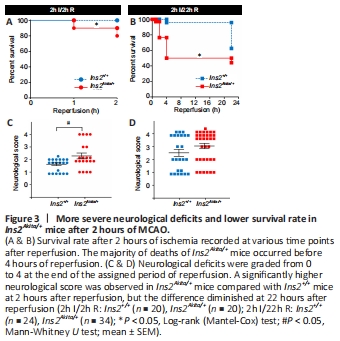
Ins2Akita/+ mice had a significantly lower survival rate than Ins2+/+ mice after reperfusion (Figure 3A and B). The majority of lethality was observed in the first 4 hours after reperfusion in Ins2Akita/+ mice, while death of Ins2+/+ mice mostly occurred later. Moreover, Ins2Akita/+ mice suffered from more severe neurological deficits at 2 hours after reperfusion (Figure 3C), whereas there was no between-group difference at 22 hours after reperfusion (Figure 3D).
Figure 4| Increased development rate of infarct area and infarct volume upon 2h I/2h R ischemic challenge in Ins2Akita/+ mice.
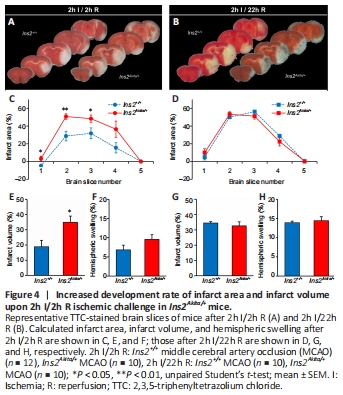
Figure 4A and B show posterior photographs of TTC-stained brain slices at 2 and 22 hours after reperfusion, respectively. At 2 hours after reperfusion, infarct areas were significantly larger in brain slices one to three of Ins2Akita/+ brains compared with Ins2+/+ brains (Figure 4C), with significantly larger infarct volume compared with Ins2+/+ brains and a trend towards greater hemispheric swelling (Figure 4E and F). However, these differences between Ins2+/+ and Ins2Akita/+ mice diminished at 22 hours after reperfusion (Figure 4D, G, and H).
Figure 5| Increased hemorrhage in Ins2Akita/+ mice as early as 2 hours after reperfusion following 2 hours of ischemia, which was further advanced at 22 hours after reperfusion.
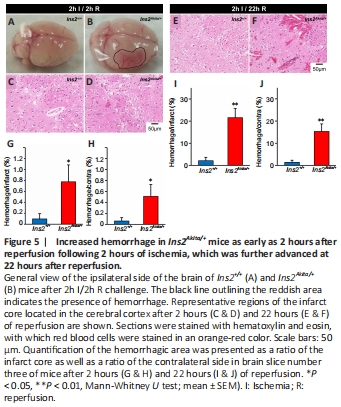
Hemorrhage (red-pink spreading) was observed in ipsilateral Ins2Akita/+ brains as early as 2 hours after reperfusion (Figure 5A and B), while eosin staining revealed its presence around blood vessels in both the infarct core (Figure 5C–F) and penumbra area (data not shown). Quantitative analysis revealed a significantly larger hemorrhagic area in brain slice number three of Ins2Akita/+ mice at 2 hours after reperfusion (Figure 5G and H), which was robustly exacerbated at 22 hours after reperfusion (Figure 5I and J).