神经损伤与修复
-
Figure 1|Comparative analysis of identified protein spots between nerve samples from the surgical repair side and nerve samples from the contralateral side.
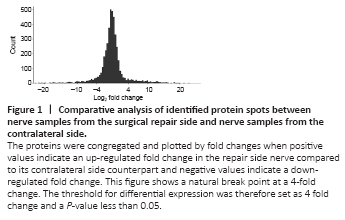
Each distal nerve proteome contained 5754 detectable proteins on the surgical side and contralateral side from both groups (immediate repair and delayed repair). To identify proteins that were differentially expressed, the relative abundance measured by mass spectrometry was compared between nerve samples (n = 8) of the surgery side from both immediate and delayed repair groups and nerve samples (n = 8) of the contralateral side from both groups. All the expression levels of such identified proteins were compared among the above groups to screen out the proteins with significant change using a P-value less than 0.05. Such proteins with a statistically significant change were further analyzed from the biological perspective to make the analysis biologically meaningful. Therefore, a fold change threshold was determined by analyzing the characteristics of protein expression profile between the operated side and contralateral side in all 8 rats, since nerve transection and repair surgery could be regarded as a notable biological factor and should lead to significant protein profile changes compared to the contralateral intact nerve. The comparative analysis between the surgery side and the contralateral side showed an apparent natural break point at a 4-fold change (Figure 1). This natural break point was probably caused by the biological factor, namely, nerve transection and repair surgery. The threshold for differential expression was therefore arbitrarily defined as a 4-fold change and a P-value less than 0.05.
Figure 3|Biological process analysis of differentially expressed proteins.
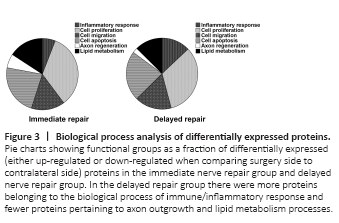
The differentially expressed proteins in either the immediate repair group or the delayed repair group were assigned to subcategories according to the biological function they are associated with. Six biological processes associated with nerve regeneration were identified: inflammatory response, cell proliferation, cell migration, cell apoptosis, axon regeneration, and lipid metabolism. Some of the proteins were involved in multiple processes and assigned to more than one process group. The percentages of differentially expressed proteins that were associated with such afore-mentioned biological processes in the immediate repair group were inflammatory response 5.89%, cell proliferation 33.89%, cell migration 15.16%, cell apoptosis 22.95%, axon regeneration 6.32% and lipid metabolism 15.78%, respectively. While, similar percentages in the delayed repair group were 12.93%, 32.93%, 16.73%, 22.59%, 2.31% and 12.52%, respectively (Figure 3). When comparing the percentages of biological processes between the immediate repair and delayed repair groups, we found that the inflammatory response process was increased in the delayed repair group, while axon regeneration processes were decreased. In the delayed repair group, 69/95 differentially expressed proteins related to the inflammatory response process were upregulated and 26/95 were down-regulated, while 6/17 differentially expressed proteins related to axon regeneration were upregulated and 11/17 were down-regulated. In the immediate repair group, 12/28 differentially expressed proteins related to the inflammatory response process were upregulated and 16/28 were down-regulated, while 14/30 differentially expressed proteins related to axon regeneration were upregulated and 16/30 were down-regulated.
Figure 4|Heatmaps of key regulatory proteins involved in the designated biological processes.
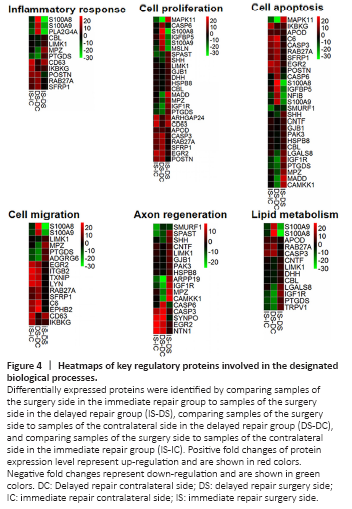
Figure 5|The impact of the key proteins on nerve regeneration.
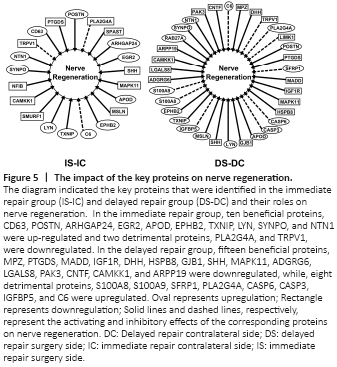
Furthermore, we analyzed the differentially expressed proteins by searching the literature to identify the proteins that are related to the key processes in nerve injury and regeneration. Multiple key proteins were identified to be involved in the inflammatory response, cell proliferation, cell apoptosis, cell migration, axon regeneration, and lipid metabolism processes (Table 3). The differential expressions of these key proteins, either up-regulation or down-regulation, in the aforementioned processes were listed in Additional Tables 1–6 and shown in the heatmap (Figure 4). By comparing fold change expression levels between surgery side samples in the immediate repair group to surgery side samples in the delayed repair group, we identified the top three differentially expressed proteins in each of the biological processes. The top three differentially expressed proteins in the inflammatory response process were S100A8, PLA2G4A, and S100A9, which were all up-regulated in the nerves that have been subjected to chronic denervation. The top three differentially expressed proteins in the cell proliferation process were S100A8, MSLN, and S100A9, which were all up-regulated in the nerves that have been subjected to chronic denervation. The top three differentially expressed proteins in the cell apoptosis process were S100A8, S100A9, and MAPK11, with MAPK11 down-regulated and the other two up-regulated in the nerves that have been subjected to chronic denervation. The top three differentially expressed proteins in the cell migration process were S100A8, S100A9, and CD63, with S100A8 and S100A9 significantly up-regulated in the nerves that have been subjected to chronic denervation. The top three differentially expressed proteins in the axon regeneration process were CAMKK1, MPZ, and IGF1R, which were all significantly down-regulated in the nerves that have been subjected to chronic denervation. The top three differentially expressed proteins in the lipid metabolism process were S100A8, S100A9, and IGF1R, with IGF1R down-regulated and the other two up-regulated in the nerves that have been subjected to chronic denervation. Among these key proteins, some were beneficial to nerve regeneration while others were detrimental. The differential expression of these beneficial or detrimental proteins might lead to the difference in nerve regeneration ability between the immediate repair and delayed repair groups. Therefore, the differential expression of proteins associated with nerve regeneration was further analyzed as shown in Figure 5. In the immediate repair group, ten beneficial proteins, namely, POSTN (Shimamura et al., 2012; Shih et al., 2014; Matsunaga et al., 2015), ARHGAP24 (Nguyen et al., 2012), SYNPO (Vlachos et al., 2009), NTN1 (Madison et al., 2000), EGR2 (Decker et al., 2006), LYN (Hossain et al., 2010), CD63 (Chernousov et al., 2013), EPHB2 (Parrinello et al., 2010), TXNIP (Sbai et al., 2010) and APOD (Ganfornina et al., 2010), and one detrimental protein C6 (Ramaglia et al., 2009) were upregulated; while eight beneficial proteins, namely, SPAST (Wood et al., 2006; Butler et al., 2010), SHH (Martinez et al., 2015), CAMKK1 (Ageta-Ishihara et al., 2009), MSLN (Roet et al., 2013), SMURF1 (Kannan et al., 2012), NFIB (Betancourt et al., 2014), PTGDS (Trimarco et al., 2014) and MAPK11 (Fragoso et al., 2003; Hossain et al., 2012), and two detrimental proteins PLA2G4A (López-Vales et al., 2008) and TRPV1 (Ren et al., 2015) were downregulated. In the delayed repair group, nine beneficial proteins, namely, RAB27A (Chen et al., 2012), NTN1 (Madison et al., 2000), SYNPO (Vlachos et al., 2009), LYN (Hossain et al., 2010), TXNIP (Sbai et al., 2010), EPHB2 (Parrinello et al., 2010), MSLN (Roet et al., 2013), APOD (Ganfornina et al., 2010) and POSTN (Shimamura et al., 2012; Shih et al., 2014; Matsunaga et al., 2015), and eight detrimental proteins, namely, S100A8 (Chernov et al., 2015), S100A9 (Chernov et al., 2015), PLA2G4A (López-Vales et al., 2008), CASP6 (Monnier et al., 2011), CASP3 (Saito et al., 2009), IGFBP5 (Simon et al., 2015), C6 (Ramaglia et al., 2009) and SFRP1 (Kele et al., 2012) were upregulated; while 15 beneficial proteins, namely, SHH (Martinez et al., 2015), LGALS8 (Pardo et al., 2019), PAK3 (Hing et al., 1999), CNTF (Sahenk et al., 1994; Newman and Verity, 1996), CAMKK1 (Ageta-Ishihara et al., 2009), MADD (Hao et al., 2010), IGF1R (Jeon et al., 2017; Joshi et al., 2015), ARPP19 (Irwin et al., 2002), MPZ (Giese et al., 1992; Lemke and Axel, 1985), PTGDS (Trimarco et al., 2014), DHH (Bajestan et al., 2006), HSPB8 (Zhang et al., 2014), GJB1 (Scherer et al., 1998), MAPK11 (Fragoso et al., 2003; Hossain et al., 2012) and ADGRG6 (Monk et al., 2011), and two detrimental proteins TRPV1 (Ren et al., 2015) and LIMK1 (Endo et al., 2003; Koch et al., 2014) were downregulated.