脑损伤
-
Figure 1|The role of heme in the secondary effects of intracerebral hemorrhage (ICH).
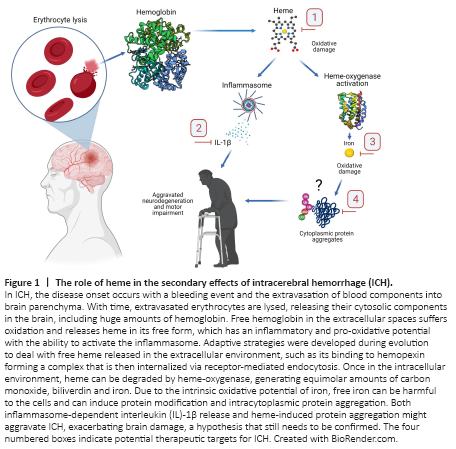
Adaptive protective mechanisms against heme toxicity and their therapeutic implications for ICH: In bleeding events, extravasated red blood cells release into the tissue huge amounts of hemoglobin, a molecule with a fundamental role in transporting O2 to the body tissues (Figure 1). In fact, this O2 binding capacity of hemoglobin is mediated by heme, which is the prosthetic group of proteins involved in numerous important biological processes. Nevertheless, in pathological events such as ICH, heme has a harmful role when released extracellularly, in part due to its redox potential. Adaptive strategies were developed by mammals to deal with free heme, contributing to its buffering and clearance. Among them are the heme-scavenger action mediated by hemopexin, which induces the internalization of heme via receptor-mediated endocytosis, and the intracellular degradation of heme by heme-oxygenase (HO), breaking heme into iron, carbon monoxide and bilirubin (Bulters et al., 2018). Iron excess is deleterious and can trigger a unique modality of cell death termed ferroptosis, which is also triggered in neurons treated with heme. To prevent the detrimental consequences of an excess of free iron, ferritin can store in the cytosol huge amounts of iron in its inert form. In line, heme detoxifying mechanisms, including those mediated by hemopexin, HO and ferritin, are extremely important for the neurological outcome in experimental models of ICH, though the efficacy of HO-1 regulators in ICH is still under debate (Bulters et al., 2018; Li et al., 2018) (Figure 1). We demonstrated that HO-1 and ferritin levels are increased in the brain after heme injection (Vasconcellos et al., 2021). Moreover, others have demonstrated an increased expression of the referred proteins in the mouse brain in standard models of ICH, as well as surrounding the hematoma in patients with ICH (Chu et al., 2018). These findings indicate that understanding the adaptive responses developed by mammals to deal with heme/iron might open new roads for the development of novel therapeutic approaches for ICH (Figure 1, boxes #1 and #3). Despite the increasing number of studies regarding the protective roles of hemopexin (Bulters et al., 2018) and HO-1 regulators (Li et al., 2018) in preclinical models of ICH, problems such as the high toxicity, low bioavailability to the brain and poor pharmacologic properties of the compounds tested still need to be overcome for future clinical trials. Iron chelators are already being tested in humans (ClinicalTrials.gov identifiers NCT02175225, NCT01662895, NCT00598572, NCT00777140, NCT02216513, NCT02875262 and NCT03754725), and the results of a recent phase 2 trial demonstrated the lack of efficacy of deferoxamine mesylate in spontaneous ICH, although this drug was shown to be safe (Selim et al, 2019). Nonetheless, little is known about the effects of iron chelators in a combined therapeutic approach, which needs further investigation.