神经退行性病
-
Figure 1|Simplified scheme of the nose-brain pathway.
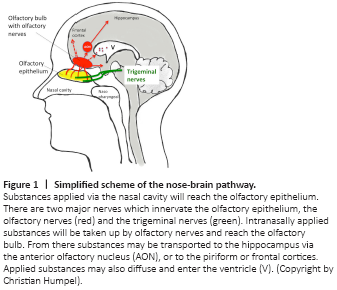
The nasal cavity is a very interesting target to infuse drugs and to allow penetration to the brain. It is easy to infuse substances into the nasal cavity and the nasal mucosa shows efficient absorption and permeability functions. A detailed and very nice review on the anatomical structure and on the transport of substances from nose-to-brain is given by G?nger and Schjindowski (2018), and a simplified scheme in Figure 1. Here I will only shortly summarize the most important issues. Nose-to-brain delivery is minimally invasive and can bypass the BBB. It has been shown that intranasal infusion is highly suitable for small molecule drugs, but also peptides or larger proteins, or viruses but also cells can pass from the nose to the brain. The nasal cavity is covered with olfactory epithelium and is innervated by nerves which project to the brain. Two important nerves innervate the olfactory epithelium, the olfactory nerves and the trigeminal nerves (Figure 1; Lee et al., 1999). The olfactory pathway targets the olfactory bulb, whereas the trigeminal pathway projects predominantly to the brain stem (Figure 1). In our recent study (Humpel, 2021), we investigated the frontal-middle-caudal cortex and thus delivery of neprilysin is considered to occur via the olfactory pathway, and indeed control substances were found in the olfactory bulb.
Figure 2|Hypothetical model of the effects of the three different intranasal neprilysin applications into transgenic Alzheimer mice.

Neprilysin is an Aβ-degrading enzyme and has a potent activity to degrade Aβ (Humpel, 2015, 2021). We have shown that neprilysin can degrade Aβ in organotypic brain slices taken from transgenic AD mice (Humpel, 2015, 2021). Very recently we showed that intranasal application of neprilysin cleared Aβ plaques in transgenic AD mice (Humpel, 2021). In this study, we injected 10 μL of neprilysin (100 ng) in both nostrils on 3 consecutive days. We used 9-month-old AD transgenic mice (APP_SweDI) which have a very high plaque load. At day 9 after intranasal neprilysin infusion, we found that the plaque load was reduced in the frontal cortex, but markedly increased in the middle and caudal cortex (Figure 2A). This suggested that infusion of pure neprilysin rapidly entered the brain, eliminated plaques quite fast, but that at later time points, this elimination caused a dramatic compensation of plaques in the cortex. The mechanism is unclear, but it seems possible that neprilysin degrades the large Aβ plaques in the brain, which then are seen as smaller Aβ plaques, or that the transgenic mice activate a compensatory process to produce more Aβ plaques.
In a previous study (Kniewallner et al., 2014), we have shown that platelets-can be loaded with NGF and provide neuroprotective activity. In our recent study (Humpel, 2021), we loaded neprilysin into platelets and infused these platelets into the nose (3 × 10 μL, bilateral, each 5 million platelets, with 40 ng neprilysin each). We show that the plaque load was reduced in the frontal cortex but not in the middle and caudal cortex (Figure 2B). Migration of cells is a well established research topic in Neuroscience, especially the transmigration of blood cells across the BBB seems to be an interesting therapeutic approach (Hohsfield and Humpel, 2015b). In our hands, we have shown that mononcytes can transmigrate across a BBB in mice and partly differentiate into a microglia-like cell type, which in turn phagocytoses and eliminates Aβ plaques (Hohsfield and Humpel, 2015a). Similarly, we hypothesized that platelets could be an interesting cell type to cross the BBB, however, we showed that most of the cells are trapped in the periphery, like spleen or lung and did not markedly enter the brain (Kniewallner et al., 2015). In the previous work (Humpel, 2021), we aimed to deliver platelets via the nose, and indeed we could show that there was some migration, but mainly in the frontal cortex/bulbus. This suggests a slow and delayed migration of cells via the olfactory pathway. It is also known that arteries run along the olfactory axon bundle (G?nger and Schjindowski, 2018), and we cannot exclude that platelets migrate along these vessels. Anyhow, we provide evidence that neprilysin can be released from platelets, migrate into the frontal cortex, and can partly eliminate some plaques. There was no evidence that plaques were removed in deeper brain areas. Definitely more work is necessary to investigate the use of blood cells as a therapeutic option via nasal application.
In the previous study (Humpel, 2021), we loaded neprilysin into collagen hydrogels, showed potent activity in vitro in brain slices and loaded the collagen hydrogels into the nose of APP_SweDI mice (3x, each 30 ng, bilateral). Our data show that neprilysin is slowly released from the collagen, is stable and causes a decrease of the plaque load in the frontal cortex but markedly stronger effects are seen in middle and caudal cortex (Figure 2C). This type of intranasal application was the most powerful one and it was surprising to see that the majority of the plaques was eliminated. We suggest that neprilysin is slowly released from the collagen in the nose and then rapidly diffuses into the caudal areas. As we injected collagen-loaded neprilysin on 3 consecutive days, this guarantees a continous slow and stable release over several days, which probably gave the optimal dose during the 9 days of experimentation. As we did not test the plaque load after 10 days, we cannot definitely say if the plaques are again compensated in the transgenic AD mouse model and increase after > 10 days. This needs to be proven in further experiments. Anyhow, independent of the outcome, we think that collagen-loaded neprilysin provides the optimal strategy to deliver an Aβ-degrading enzyme deep into the brain to clear plaques.