神经退行性病
-
Figure 1|Effects of voluntary exercise on cognitive function in 5- and 9-month-old APP/PS1 mice with or without AQP4.

Compared with male littermates of the same age, AD-like pathological changes in female mice were milder, and the individual differences were more obvious. Accordingly, only male mice were used in the present study. AQP4–/–/APP/PS1 mice were generated by crossing APPswe/PS1-dE9 mice (Jackson Laboratories, Philadelphia, PA, USA) with AQP4–/– mice that were established in our laboratory (Fan et al., 2005), as described previously (Xu et al., 2015). We randomly divided 3- and 7-month-old male AQP4–/–/APP/PS1 mice, APP/PS1 mice, and their wild-type (WT) littermates (license No. SCXK (Su) 2021-0001; Animal Core Facility of Nanjing Medical University, Nanjing, China) into exercise and sedentary groups (n = 12–13 per group), and then exposed those in the exercise groups to 2 months of voluntary exercise followed by behavioral testing (Figure 1A). The mice were housed 3 or 4 per cage in a room with a constant room temperature (18–22°C), a relative humidity of 30–50%, and controlled illumination (12:12 hours light/dark cycle). Food and water were available ad libitum. The animal experiments were approved by the Institutional Animal Care and Use Committee of Nanjing Medical University (No. IACUC-1912002) in November 2019. All experiments were designed and reported according to the Animal Research: Reporting of In Vivo Experiments (ARRIVE) guidelines (Percie du Sert et al., 2020).
We assessed spatial learning and memory in mice using the MWM test. For the 5-month-old group, voluntary exercise reduced the escape latency in WT mice (F(1, 137) = 59.56, P < 0.0001) and APP/PS1 mice (F(1, 132) = 39.16, P < 0.0001), but not AQP4–/–/APP/PS1 mice (F(1, 134) = 0.9870, P = 0.3223; Figure 1B). For the 9-month-old group, exercise shortened the escape latency in WT mice (F(1, 143) = 36.81, P < 0.0001), but not in APP/PS1 mice (F(1, 139) = 0.6231, P = 0.4312) or AQP4–/–/APP/PS1 mice (F(1, 136) = 0.2689, P = 0.6049; Figure 1C). The time spent in the target quadrant and the number of platform crossings during the probe trials on day 7 were highest in the 5-month-old WT mice (target quadrant: P = 0.0213, number of platform crossings: P = 0.02, WT-Exe vs. WT-Con, respectively) and APP/PS1 mice in the voluntary exercise group (target quadrant: P = 0.0429, number of platform crossings: P = 0.0442, APP/PS1-Exe vs. APP/PS1-Con, respectively). For the 9-month-old group, the above parameters were only increased in the WT mice exposed to the exercise intervention (target quadrant: P = 0.0126; number of platform crossings: P = 0.027, WT-Exe vs. WT-Con, respectively). Notably, exercise did not improve spatial memory performance in the 5-month-old or 9-month-old AQP4–/–/APP/PS1 mice (5-month-old: target quadrant: P = 0.6146, number of platform crossings: P = 0.9853; 9-month-old: target quadrant: P = 0.7589, number of platform crossings: P = 0.9439, AQP4–/–/APP/PS1-Exe vs. AQP4–/–/APP/PS1-Con, respectively; Figure 1D and E).
We performed the Y-maze test to measure short-term working memory. For the 5-month-old group, compared with the corresponding sedentary controls, both WT and APP/PS1 mice in the exercise group exhibited higher percentages of time spent in the novel arm (P = 0.0225, WT-Exe vs. WT-Con; P = 0.0324, APP/PS1-Exe vs. APP/PS1-Con) and a higher number of entrances into the novel arm (P = 0.00146, WT-Exe vs. WT-Con; P = 0.0172, APP/PS1-Exe vs. APP/PS1-Con). Nevertheless, the performance of the AQP4–/–/APP/PS1 mice was similar between the exercise and sedentary groups (time spent: P = 0.9174; number of entrances: P = 0.9610, respectively). For the 9-month-old group, exercise increased the amount of time spent in the novel arm and the number of entrances of the WT mice (time spent: P = 0.03; number of entrances: P = 0.0211, vs. WT-Con, respectively), but failed to reverse working memory deficits in APP/PS1 mice (time spent: P = 0.8324; number of entrances: P = 0.7561, vs. APP/PS1-Con, respectively) and AQP4–/–/APP/PS1 mice (time spent: P = 0.9285; number of entrances: P > 0.9999, vs. AQP4–/–/APP/PS1-Con, respectively; Figure 1F and G).
Figure 2|Effect of voluntary exercise on glymphatic transport in 5- and 9-month-old APP/PS1 mice with or without AQP4.
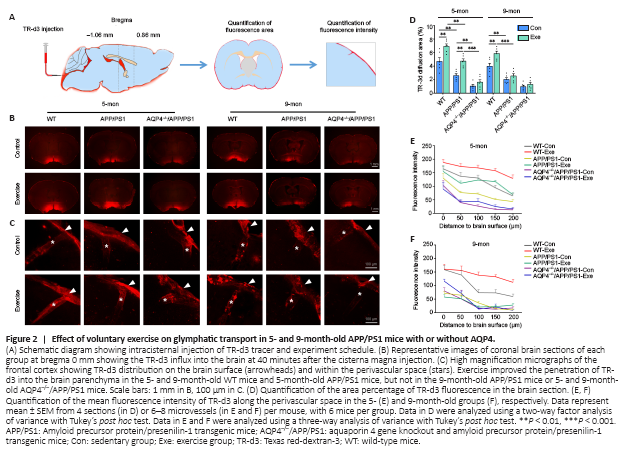
We assessed glymphatic transport by quantifying TR-d3 penetration into the brain parenchyma following a cisterna magna injection (Figure 2A). We found that APP/PS1 mice had a smaller percentage of the TR-d3-positive area and a decreased paravascular fluorescence intensity compared with WT mice at the age of 5 months (area percentage: P = 0.0042; fluorescence intensity: F(1, 29) = 42.98, P < 0.0001) and 9 months (area percentage: P = 0.0022; fluorescence intensity: F(1, 30) = 91.07, P < 0.0001, respectively). Both 5- and 9-month-old WT mice exposed to voluntary exercise exhibited an increased fluorescence penetration area (5 months: P = 0.0022; 9 months: P = 0.0029, vs. control, respectively) and an increased intensity (5 months: F(1, 28) = 39.05, P < 0.0001; 9 months: F(1, 30) = 21.12, P < 0.0001, vs. control, respectively). However, for APP/PS1 mice, the beneficial effect of exercise on penetration of TR-d3 into the brain parenchyma was observed in the 5-month group only (area percentage: P = 0.0033; fluorescence intensity: F(1, 29) = 39.27, P < 0.0001, vs. control, respectively). Neither 5- nor 9-month-old AQP4–/–/APP/PS1 mice treated with exercise showed an increase in the percentage of the TR-d3 positive area (5 months: P = 0.8651; 9 months: P = 0.9786) or the paravascular fluorescence intensity (5 months: F(1, 30) = 0.3178, P = 0.5771; 9 months: F(1, 30) = 1.693, P = 0.2031; Figure 2B–F and Additional Figure 3). These results suggest that the ameliorating effect of exercise on glymphatic transport malfunction in APP/PS1 mice is timing-dependent, and that AQP4 deletion eliminates the beneficial effect of exercise on glymphatic transport.
Figure 3|Effects of voluntary exercise on Aβ accumulation in 5- and 9-month-old APP/PS1 mice with or without AQP4.
We further evaluated whether voluntary exercise offered at different time points had unique effects on the reduction of the brain Aβ burden in APP/PS1 mice. Compared with age-matched APP/PS1 mice, both 5- and 9-month-old AQP4–/–/APP/PS1 mice showed an increased Aβ load in the hippocampus and frontal cortex, as revealed by quantification of the area percentage and number of thioflavine-S positive fibrillar plaques and 6E10-immunopositive diffuse plaques (all P < 0.05). Exercise decreased Aβ plaque accumulation in the above two brain regions in APP/PS1 mice at 5 months (all P < 0.05 for the above indexes). We did not observe a beneficial effect of exercise on Aβ load in the AQP4–/–/APP/PS1 mice at either of the two different time points (all P > 0.05; Figure 3A–F). Consistent with this, the Western blot and ELISA revealed that voluntary exercise only decreased Aβ1–40 and Aβ1–42 levels in the forebrain samples of 5-month-old APP/PS1 mice (all P < 0.05) rather than the 9-month-old APP/PS1 mice (all P > 0.05). Voluntary exercise failed to decrease brain Aβ1–40 and Aβ1–42 levels in both 5- and 9-month-old AQP4–/–/APP/PS1 mice (all P > 0.05; Figure 3G–I and Additional Figure 4A and B). The above data indicate that voluntary exercise reduces brain Aβ load in APP/PS1 mice in a timing-dependent manner, and that this effect is reliant on the presence of AQP4.
Figure 4|Effects of voluntary exercise on reactive gliosis in the hippocampus of 5- and 9-month-old APP/PS1 mice with or without AQP4.
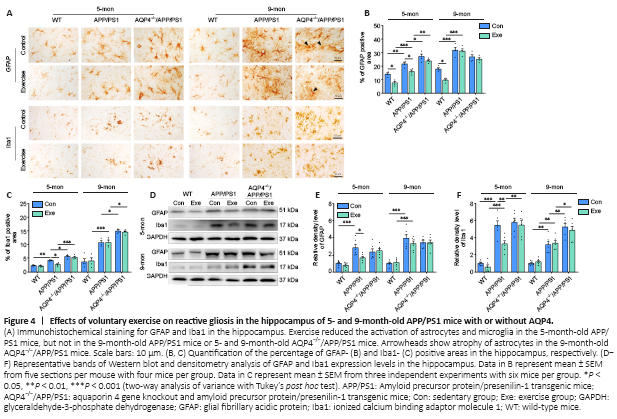
In addition to Aβ deposition, reactive gliosis is a pathological hallmark of AD (Lopategui Cabezas et al., 2014; Acosta et al., 2017). We determined the effects of voluntary exercise and/or AQP4 deletion on the activation of astrocytes and microglia in APP/PS1 mice at different stages of AD-like progression. Both GFAP-positive astrocytes and Ibal-positive microglia were activated in the frontal cortex and hippocampus of APP/PS1 mice at 5 months old, and this was ingreasingly apparent at 9 months old. AQP4 deletion in 5-month-old APP/PS1 mice mildly increased the activation of microglia and astrocytes in the frontal cortex and hippocampus. Microglia activation was also more pronounced in AQP4–/–/APP/PS1 mice at 9 months, as revealed by the high percentages of Iba1 positive areas in the frontal cortex (P = 0.0011) and hippocampus (P = 0.0168) compared with that in age-matched APP/PS1 mice. However, a considerable proportion of astrocytes underwent atrophy, characterized by a breakdown of the cell body with residual processes, in 9-month-old AQP4–/–/APP/PS1 mice (Figure 4A and Additional Figure 5A). There was no overall change in immunohistochemical labeling patterns of GFAP or Ibal in the frontal cortex and hippocampus in 5- and 9-month-old WT-exercise mice compared with that in age-matched sedentary controls. As for APP/PS1 mice, exercise reduced the percentages of the positive areas for GFAP (cortex: P = 0.0416; hippocampus: P = 0.0331) and Iba1 (cortex: P = 0.0027; hippocampus: P = 0.0107) in the 5-month-old group but not the 9-month-old group (GFAP: cortex, P = 0.9704; hippocampus, P = 0.9993; Iba-1: cortex, P = 0.9887; hippocampus, P > 0.9999). Exercise did not mitigate the reactivation of astrocytes or microglia in AQP4–/–/APP/PS1 mice in either age group (5 months: GFAP: cortex, P = 0.9991; hippocampus, P = 0.3894; Iba-1: cortex, P > 0.9999; hippocampus, P = 0.9745; 9 months: GFAP: cortex, P = 0.5592; hippocampus, P = 0.9685; Iba-1: cortex, P = 0.9579; hippocampus, P = 0.9994; Figure 4B and C and Additional Figure 5B). The Western blot results also showed that the AQP4 deficiency eliminated the time-dependent effects of voluntary exercise on GFAP and Ibal expression levels in the hippocampus of APP/PS1 mice (5 months: GFAP: P = 0.9986; Iba-1: P = 0.9931; 9 months: GFAP: P > 0.9999; Iba-1: P = 0.9602; Figure 4D–F). Consistent with this, voluntary exercise partially reduced neuroinflammatory factor levels in APP/PS1 mice at 5 months (IL-1β: P = 0.0121; IL-6: P = 0.0184; TNF-α: P = 0.0191), but not at 9 months old (IL-1β: P = 0.9883; IL-6: P > 0.9999; TNF-α: P = 0.9964). The AQP4 deletion in APP/PS1 mice completely abolished the mitigating effect of the exercise intervention on neuroinflammation (5 months: IL-1β: P = 0.8353; IL-6: P = 0.7361; TNF-α: P = 0.9959; 9 months: IL-1β: P = 0.9966; IL-6: P = 0.7856; TNFα: P = 0.9983; Additional Figure 5C). The above results indicate that voluntary exercise ameliorates reactive gliosis and neuroinflammation in the onset stage but not the mid-stage of APP/PS1 mice, and that this timing effect is absent in AQP4–/–/APP/PS1 mice.
Figure 5|Effects of voluntary exercise on AQP4 expression and polarity in the frontal cortex in 5- and 9-month-old APP/PS1 mice as revealed by AQP4 and Congo-red double staining.
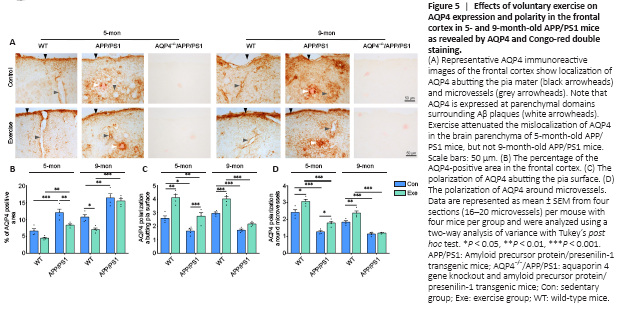
Substantial evidence suggests that reactive astrogliosis results in the mislocalization of AQP4 from the endfeet to the entire cell surface of astrocytes, subsequently contributing to dysfunctional glymphatic clearance (Iliff et al., 2012; Kress et al., 2014; Wang et al., 2019). Here, immunohistochemical staining revealed that AQP4 expression selectively bordered the subarachnoid space and microvessels with very low intensity in the adjacent brain parenchyma in WT mice. AQP4 was abnormally expressed at the non-vascular domains, especially those surrounding Congo-red positive plaques, in 5-month-old APP/PS1 mice (Figure 5A). The mislocalization of AQP4 was further discernible in the brain parenchyma of 9-month-old APP/PS1 mice, causing a total loss of AQP4 polarity. Voluntary exercise decreased AQP4 expression at the non-vascular domains in 5-month-old APP/PS1 mice but not 9-month-old APP/PS1 mice. Semi-quantitative analysis demonstrated that voluntary exercise decreased the percentage of the AQP4-positive area (P = 0.0078) and improved AQP4 polarity around microvessels (P = 0.0398) and pia mater (P = 0.0148) in 5-month-old APP/PS1 mice exposed to exercise. In contrast, these changes were not apparent in 9-month-old APP/PS1 mice exposed to exercise (percentage of the AQP4-positive area: P = 0.8609; AQP4 polarization around microvessels: P = 0.9586; AQP4 polarization around pia surface: P = 0.0944; Figure 5B–D). Double immunofluorescence labeling for GFAP and AQP4 further confirmed the presence of activated astrocytes with impaired AQP4 polarization in the brain parenchyma of both 5- and 9-month-old APP/PS1 mice, indicating that voluntary exercise ameliorated the mislocalization of astrocyte AQP4 in the onset stage but not mid-stage of progression in APP/PS1 mice (Additional Figure 6A and B).
Figure 6|Effects of voluntary exercise on BDNF-TrkB signaling activation and synaptic protein levels in the hippocampus of 5- and 9-month-old APP/PS1 mice with or without AQP4.
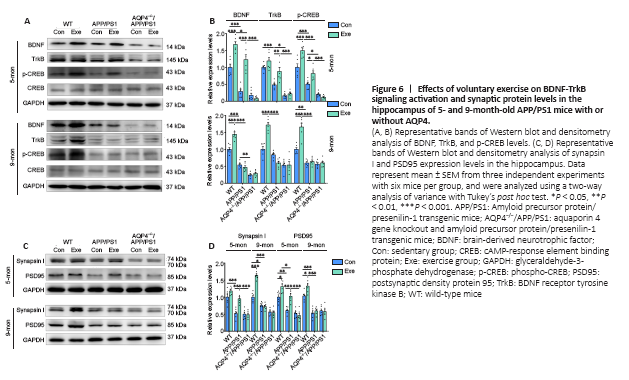
The BDNF signaling pathway is associated with synaptic function and regulates the expression of synaptic proteins (Poo, 2001; Chao, 2003). Down-regulation of the BDNF signaling pathway and synaptic protein loss occur in AD patients (Peng et al., 2005; Kim et al., 2017) as well as animal models of AD (Poon et al., 2011; Bartolotti et al., 2016). Moreover, the BDNF signaling pathway is involved in the neuroprotective effects of exercise in AD (Vaynman et al., 2004; Liu and Nusslock, 2018), stroke (Ferrer et al., 2001; Liu et al., 2020), and depression (Duman and Monteggia, 2006; Martinowich et al., 2007). The present study measured the activation of the BDNF-TrkB-CREB pathway and the expression levels of PSD95 and synapsin I in the forebrain. We found that the expression levels of BDNF (mature form), TrkB, and p-CREB generally decreased in APP/PS1 mice compared with those in WT controls at 5 (BDNF: P < 0.0001; TrkB: P = 0.0004; p-CREB: P = 0.0002, respectively) and 9 months old (BDNF: P < 0.0001; TrkB: P = 0.6340; p-CREB: P = 0.0014, respectively). Voluntary exercise activated this signaling pathway in WT mice aged 5 (BDNF: P = 0.0001; TrkB: P = 0.4700; p-CREB: P = 0.0002, respectively) and 9 months old (BDNF: P < 0.0001; TrkB: P < 0.0001; p-CREB: P < 0.0001, respectively), but this was only the case in APP/PS1 mice at 5 months old (BDNF: P < 0.0001; TrkB: P = 0.0090; p-CREB: P = 0.0329, respectively; Figure 6A and B). Consistent with this, voluntary exercise significantly up-regulated synapsin I and PSD95 levels in the hippocampus of 5-month-old WT and APP/PS1 mice (all P < 0.05), but not AQP4–/–/APP/PS1 mice (all P > 0.05), as revealed by immunohistochemical staining and Western blot analysis. For the 9-month-old group, a beneficial effect of voluntary exercise on synaptic protein levels was only observed in WT mice (immunohistochemistry: synapsin I: P = 0.0146; PSD95: P = 0.0494; Western blot: synapsin I: P < 0.0001; PSD95: P = 0.0309, respectively; Figure 6C and D and Additional Figure 7A–C). Together, these results indicate that voluntary exercise improves BDNF signaling activation and synaptic plasticity in the hippocampus of APP/PS1 mice in a timing-dependent manner, which is eliminated in the absence of AQP4.