脊髓损伤
-
Figure 1|An overview of the properties of cryogels that make them useful biomaterials for neuroscience applications.
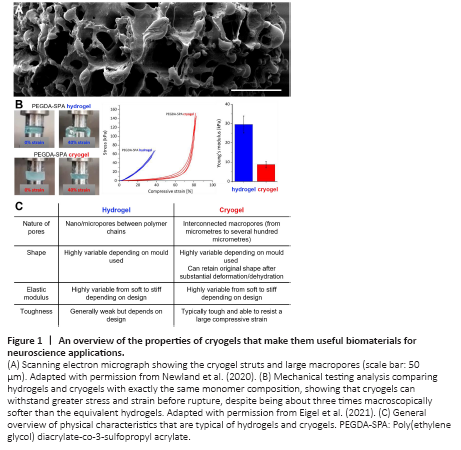
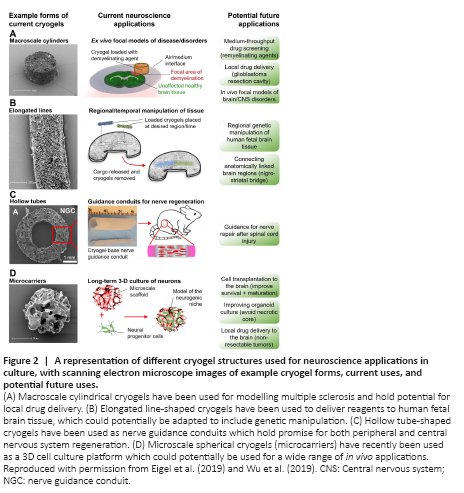
Figure 2|A representation of different cryogel structures used for neuroscience applications in culture, with scanning electron microscope images of example cryogel forms, current uses, and potential future uses.
Cryogels are similar to their hydrogel counterparts in that they are composed of a 3-dimensional hydrophilic network. However, an important difference is their macroporous structure, with comparatively large open pores surrounded by thin struts of hydrogel (Figures 1A and 2). This structure is obtained by freezing the hydrogel precursor solution prior to (or during) crosslinking, forcing the polymer network to form around the ice crystals (Eigel et al., 2021a). The sponge-like morphology of cryogels makes them tough, due to the condensed polymer network found in the struts. However, macroscopically they are soft and can be substantially compressed (Figure 1B), reforming to their original size and shape once the compressive force is removed (Bencherif et al., 2012). This is an important characteristic that enables these cryogels to be inserted and then later removed from CNS tissue (Eigel et al., 2021b).
This mechanical toughness has enabled these macroscale cryogels to be easily used to deliver reagents to regional areas of tissue in culture and improve models of CNS injury. For example, cylinder-shaped cryogels were used to create a focal ex vivo model of demyelination (Eigel et al., 2019). These cryogels were dipped in a demyelinating agent, lysophosphatidylcholine, and then placed next to brain or spinal cord tissue slices in culture. As depicted in Figure 2A, this created a small region of demyelination surrounded by healthy tissue (Eigel et al., 2019). Aside from their ease of use, the cryogels also provided the important advantage of more accurately reproducing the patchy demyelination pathology observed in multiple sclerosis in humans (Eigel et al., 2019), in comparison to pre-existing “global demyelination” models (Zhang et al., 2011). This patchy demyelination allowed the role of the infiltrating glial cells, from the surrounding unaffected areas, in remyelination to be analyzed. Another advantage of this focal demyelination approach is that it can also be applied to create focal regions of demyelinated grey matter in vivo (Zoupi et al., 2021). This has allowed researchers to unpick mechanisms of demyelination vs. inflammation in neural degeneration, and highlight the selective vulnerability of specific neuronal types to degeneration following demyelination (Zoupi et al., 2021). The ability of the cryogels to improve the replication of patient pathology in these models highlights their potential to be further developed as a platform for analyzing therapeutics for remyelination.
The mechanical toughness of cryogels has also proved useful when working with human brain tissue cultures. Cryogels generated using 3D printed templates have allowed researchers to label specific regions of human fetal brain tissue slices with fluorescent dyes, enabling specific cells to be identified and followed in culture (Eigel et al., 2021b). They have also been used to transiently apply a neurotoxin to reversibly and repeatedly inhibit neuron activity in a spatiotemporal manner within a brain tissue slice (Eigel et al., 2021b). Human tissue cultures lack many of the genetic tools available in animal models, but we speculate that cryogels could also be used to deliver genetic material to this tissue, either through viral vectors or non-viral constructs. A notable advantage of the cryogels for such applications is that as they are typically formed within a template, meaning that they can be created to almost any size or shape whilst retaining their properties post-compression (Bencherif et al., 2012). This has allowed the formation of high aspect ratio (elongated) line-shaped cryogels, which, despite narrow widths down to 150 μm, are strong enough to be handled with forceps, loaded with reagents and placed onto brain tissue slices (Figure 2B) (Eigel et al., 2021b; Ucar et al., 2021). This would enable cryogels to be made to order and reproducibly target a specified area of the brain tissue slice. This characteristic, alongside their ability to be removed without damaging the tissue (Eigel et al., 2021b), gives them a major advantage over conventional hydrogels, as those formed from the equivalent monomers/solid content cannot be handled without breaking (Eigel et al., 2021b; Figure 1C). Overall, cryogels have shown promise for use as biocompatible tools to deliver a wide range of cargos to neural tissue cultures, which could include CRISPR/cas9 systems, therapeutics and even cells.
One characteristic of cryogels that makes them suitable tools for cell delivery is that the pore size of the scaffold can easily be adjusted by varying the freezing regime used (Eigel et al., 2021a). Slower freezing rates yield larger pores at sizes suitable for cell adherence and growth within the cryogel scaffold itself (Figure 2D) (Newland et al., 2020). Indeed, pores can be made sufficiently large enough to allow infiltration of the neural progenitor cells throughout the cryogel structure within the first three days of culture (Newland et al., 2020). When used as a 3D cell culture system, these cryogels allowed 28 days of neural progenitor cell culture without the formation of a necrotic core or spontaneous differentiation, two commonly occurring problems of 3D neurosphere cultures. In principle, this culture technique could be adapted to organoid cultures, potentially allowing organoids to retain a viable core as they grow.The ability of cryogels to deliver reagents, proteins and cells makes them an attractive starting point for the design of soft implantable therapeutic drug delivery systems. For example, the resection cavity left after glioblastoma surgery could be filled with soft cryogel delivery systems, allowing repurposing of drugs that cannot pass the blood-brain barrier. Combining imaging of the tumor with 3D printing technology to create molds, cryogels of a particular size and shape could be prepared to approximately match that of the cavity. Furthermore, charged cryogels could be electrostatically loaded with a precise amount of oppositely charged chemotherapeutics (e.g., doxorubicin). Filling the resection cavity with this drug loaded cryogel could potentially achieve an even and sustained release of these therapeutics to all surfaces of the resection cavity. Alternatively, injectable microscale cryogels (such as those shown in Figure 2D) may be useful for local intratumoral delivery of therapeutics to non-resectable tumors, provided that they can still be reached by stereotactic injection. Cryogels, with their unique combination of high elasticity, high toughness and macroporosity, are well-suited to applications such as nerve guidance conduits, giving mechanical support to regenerating neurons without hindering fluid flow to these cells (Figure 2C) (Wu et al., 2019).
点击此处查看全文