脑损伤
-
Figure 1|Identification of exosomes derived from bone marrow mesenchymal stem cells.
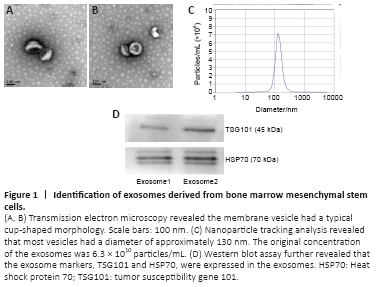
Exosomes were isolated from bMSCs using ultracentrifugation and identified using transmission electron microscopy, nanoparticle tracking analysis, and western blot analysis. A typical cup-shaped membrane vesicle morphology was observed (Figure 1). The size distribution profiles from the nanoparticle tracking analysis revealed that most vesicles had a diameter of ~130 nm. The original concentration of exosomes was 6.3 × 1010 particles/mL (exosomes detected were diluted by 1000-fold). Western blotting analysis further revealed that exosome markers TSG101 and HSP70 were expressed in the exosomes (Figure 1).
Figure 2|Effects of bMSCs and exosomes on the phenotype of activated BV2 microglia.
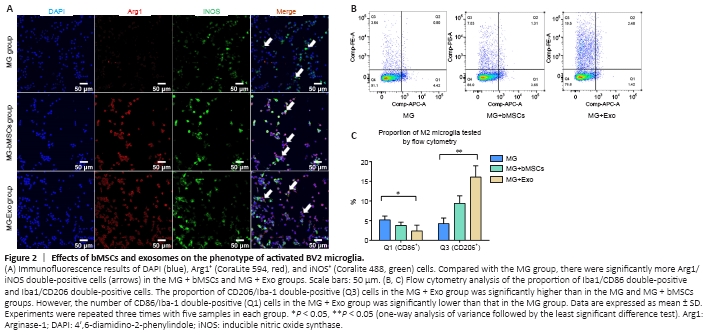
After BV2 microglia cells were activated, they were then co-cultured with bMSCs or exosomes for 48 hours to facilitate the polarization of activated BV2 cells toward the anti-inflammation type (Figure 2A). This was performed to investigate the effects of bMSCs and bMSC-derived exosomes on the polarization phenotypes of activated BV2 cells in a co-culture system. The expression of the anti-inflammation marker, Arg1, was increased significantly in the MG + bMSCs and MG + Exo groups, and the expression of pro-inflammation marker iNOS was decreased in the MG + bMSCs and MG + Exo groups. Flow cytometry analysis was then performed to determine the proportion of pro-inflammatory and anti-inflammatory phenotype cells (Figure 2B). Activated BV2 cells were transformed to the anti-inflammatory phenotype after 48 hours of co-culturing with bMSCs or exosomes. Moreover, the exosomes were associated with a stronger anti-inflammatory effect (Figure 2C).
Figure 4|Exosomes induce reduction of the injured area and cerebral cell apoptosis in the lesion areas of TBI mice.
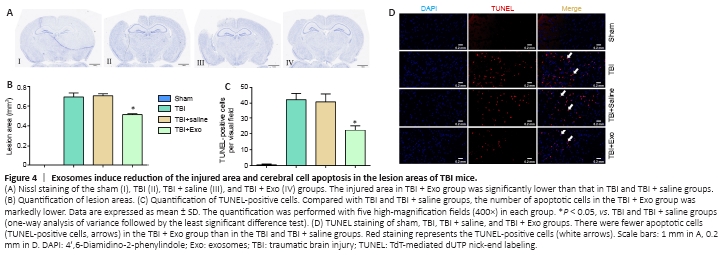
The lesion area and apoptotic cerebral cortical cells were determined 7 days after TBI to detect the effects of exosomes on the nervous system post-TBI in vivo (Figure 4). The damaged area in mice in the TBI + Exo group was smaller than that in the TBI and TBI + saline groups (Figure 4A and B). Brain sections were then subjected to TUNEL staining followed by quantification of TUNEL-positive cells. The TBI + Exo group had significantly fewer apoptotic neurons than did the TBI and TBI + saline groups (Figure 4C and D).
Figure 5|Effects of exosomes on neuroinflammation of injured area in TBI mice.

An in vivo experiment was designed in which exosomes were injected through the tail vein followed by induction of the TBI model to examine whether exosomes could inhibit inflammation in the central nervous system after induction of TBI. The expression levels of Arg1, iNOS, and inflammatory factors were then detected using immunofluorescence, western blot, and real-time quantitative polymerase chain reaction (Figure 5). The proportion of Arg1-positive cells in the TBI + Exo group was significantly higher than that in the other groups (Figures 5A and B). In the same line, according to the western blot results, the protein expression level of Arg1 in the TBI + Exo group was significantly higher than that in the other groups. However, the expression of iNOS protein in the TBI + Exo group was significantly lower than that in the TBI and TBI + saline group (P < 0.05; Figure 5C). Figure 5DI–III shows the mRNA expression levels of the related inflammatory factors 1, 3, and 7 days, respectively, after the onset of TBI. Evidently, the mRNA expression levels of IL-10 and TGF-β in the TBI + Exo group were higher than those in the TBI and TBI + saline groups on days 1, 3, and 7 post-TBI. Moreover, the mRNA expression levels of IL-1β and TNF-α in the TBI + Exo group were lower than those in the TBI and TBI + saline groups on days 3 and 7 post-TBI. Injection of exosomes upregulated the expression of STAT3 but inhibited the expression of nuclear factor-kappa B (NFκB) 7 days post-TBI (Figure 5D [IV]).
Figure 7|The effect of different expression levels of miR-181b on cerebral cell apoptosis and neuroinflammation in injured area of TBI mice.

Lentiviral vectors were used to obtain different expression levels of miR-181b to verify the role of miR-181b in the neuroinflammation process post-TBI. The expression level of miR-181b was detected 7 days after transfection of the mice with the lentivirus. Cell apoptosis detected in each group on day 7 post-TBI revealed that the number of TUNEL-positive cells in the miR-181b upregulation group was significantly lower than that in the other two groups (Figures 7A and B). The expression level of miR-181b in the upregulation and downregulation group was 2.7 times higher and 0.4 times lower than that in normal C57BL/6J mice, respectively (Figure 7C). The microglial markers, Arg1 and iNOS, and the transcriptional regulator, STAT3, were detected using western blot (Figure 7D). The TBI-up group had a higher expression level of Arg1 and STAT3, but a lower expression level of iNOS than did the normal and TBI-down groups. Inflammatory factors were also detected on day 1, 3, and 7 post-TBI (Figure 7E [I–III]). The mRNA expression level of IL-10 and TGF-β in the TBI-up group was significantly higher than that in the other two groups on day 1, 3, and 7 post-TBI (P < 0.05). However, the mRNA expression level of IL-1β in the TBI-up group was significantly lower than that in the other two groups on day 1, 3, and 7 post-TBI (P < 0.05). Similarly, the mRNA expression level of TNF-α in the TBI-up group was significantly lower than that in the other two groups on day 3 and 7 post-TBI (P < 0.05). Nonetheless, the expression level of IL-6 mRNA was not significantly different between groups on day 1, 3, and 7 post-TBI. Further analysis of the TBI-up group revealed that the expression levels of IL-10 and TGF-β mRNA gradually increased after TBI (Figure 7E [IV]).