脑损伤
-
Figure 2| Effect of maraviroc on microglial activation and polarization in the perilesional cortex area of mice after traumatic brain injury.
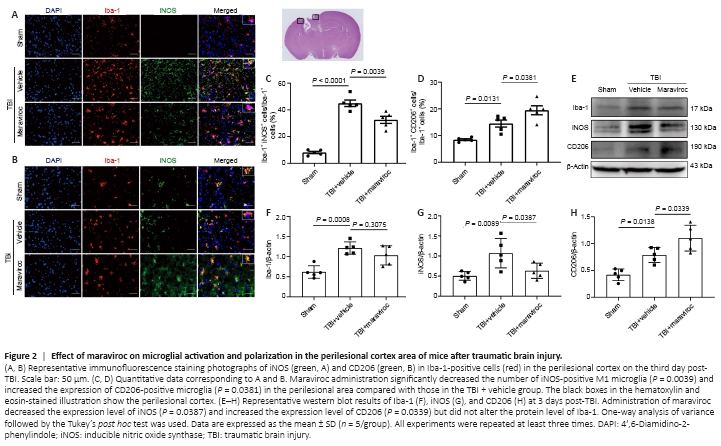
Microglia convert from the resting type into the M1 and M2 phenotypes after TBI; this process plays a vital role in the neuroinflammatory response (Long et al., 2020). To determine the effect of maraviroc on microglial polarization, coimmunofluorescence staining of a panmicroglial marker (ionized calcium-binding adapter molecule 1 [Iba-1]), an M1 microglial marker (inducible nitric oxide synthase [iNOS]), and an M2 microglial marker (macrophage mannose receptor 1 [CD206]) was performed to determine the shifts in microglial polarization 3 days after TBI. Maraviroc administration remarkably decreased the number of Iba-1-positive cells that also expressed iNOS and increased the expression of CD206 in the perilesional area, indicating an M1-to-M2 microglial transition (Figure 2A–D). Furthermore, western blot analysis at 3 days post-TBI in the lesioned cortex showed that iNOS expression was inhibited and CD206 expression was significantly increased after maraviroc administration. However, the protein expression level of Iba-1 in tissues from mice in the maraviroc treatment group did not significantly differ from that in mice in the vehicle treatment group (Figure 2E–H).
Figure 3| Effect of maraviroc on neutrophil and macrophage infiltration after traumatic brain injury.
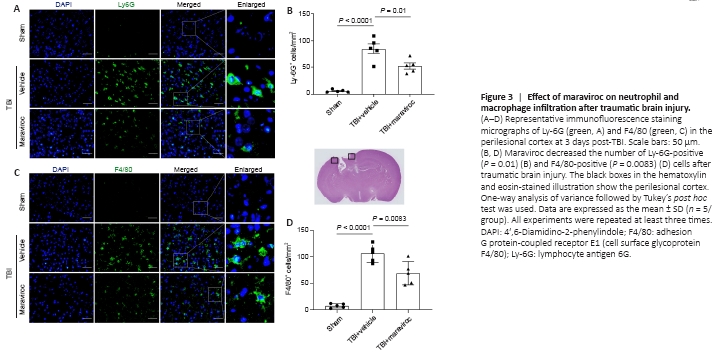
In addition, immunofluorescence staining showed the accumulation of adhesion G protein-coupled receptor E1 (cell surface glycoprotein F4/80 [F4/80])-positive and lymphocyte antigen 6G (Ly-6G)-positive cells in the pericontusional region in the TBI + vehicle group compared with that in the TBI + maraviroc group at 3 days post-TBI (Figure 3A–D).
Figure 4| Effect of maraviroc on the HMGB1/NF-κB pathway and inflammatory cytokines in the perilesional cortex after traumatic brain injury.
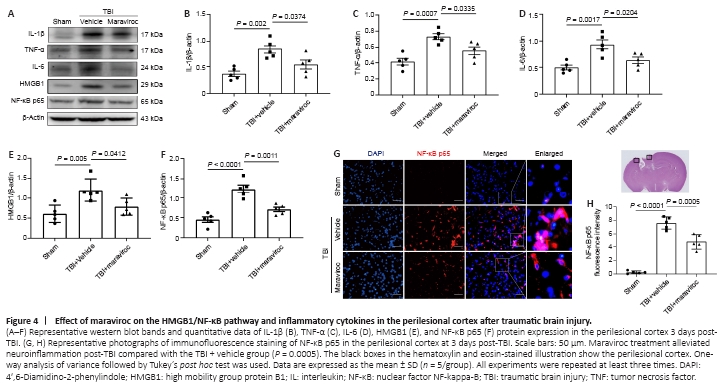
High mobility group protein B1 (HMGB1) translocation and release have been shown to activate microglia and exacerbate neuroinflammation induced by TBI (Paudel et al., 2020). The nuclear factor kappa B (NF-κB) pathway is related to high expression levels of HMGB1 and the subsequent release of inflammatory factors. The HMGB1/NF-κB pathway may play a critical role in the pathological process of TBI (Chen et al., 2018). Western blot analysis illustrated that the expression levels of HMGB1 and NF-κB p65 in the lesioned cortex were significantly increased at 3 days after TBI (Figure 4A, E, and F). In contrast, administration of maraviroc effectively decreased HMGB1 and NF-κB p65 protein expression compared with vehicle treatment after TBI. Moreover, a western blot assay of proinflammatory cytokine levels showed that maraviroc treatment significantly inhibited the expression of these inflammatory factors compared with vehicle treatment after TBI (Figure 4A–D). Immunofluorescence staining further demonstrated that the TBI + maraviroc group had a significantly reduced percentage of cells with nuclei that stained positive for NF-κB p65 compared with that in the TBI + vehicle group (Figure 4G and H).
Figure 5|Effect of maraviroc on the expression of the NLRP3 inflammasome after traumatic brain injury.

Western blotting and immunofluorescence were conducted to determine the NLRP3 inflammasome expression levels among different groups. The TBI + vehicle group had elevated levels of NLRP3, caspase-1 p20, apoptotic speck-containing protein, IL-18, IL-1β, and gasdermin-D compared with the sham group (Figure 5A–G). The TBI + maraviroc group had significantly decreased protein levels of the NLRP3 inflammasome compared with the TBI + vehicle group. In addition, immunofluorescence analysis revealed that the elevated caspase-1 p20 immunoreactivity in the pericontusional cortex was greatly alleviated by maraviroc administration compared with vehicle administration (Figure 5H and I).
Figure 6|Effect of maraviroc on neurotoxic reactive astrocyte activation in the pericontusional cortex of mice with traumatic brain injury.
We estimated whether maraviroc treatment suppressed neurotoxic reactive astrocytes using immunohistochemical and western blot assays. The expression levels of glial fibrillary acidic protein (GFAP) were significantly increased in the TBI + vehicle group in the ipsilateral hemisphere compared with the sham group (P = 0.0091), but there was no difference between the TBI + vehicle group and the TBI + maraviroc group (P = 0.3987; Figure 6A and B). However, we observed that the protein levels of the neurotoxic reactive astrocyte-associated marker complement C3 were elevated in the vehicle-treated group compared with the sham group and decreased after maraviroc administration (Figure 6C). Coimmunofluorescence revealed that the maraviroc treatment group exhibited a notable decline in the number of C3-positive and GFAP-positive astrocytes compared with the vehicle treatment group in the lesioned cortex 3 days post-TBI (Figure 6D and E).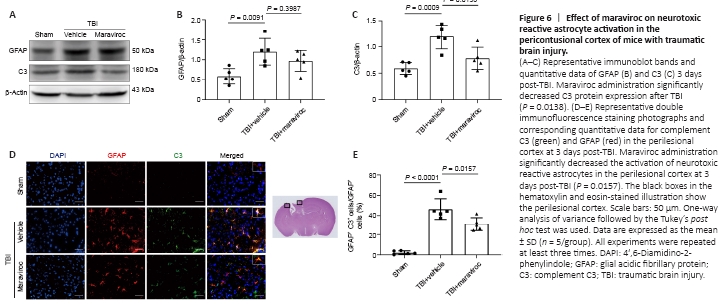
Figure 7|Effect of maraviroc on neuronal apoptosis at 3 days after traumatic brain injury.
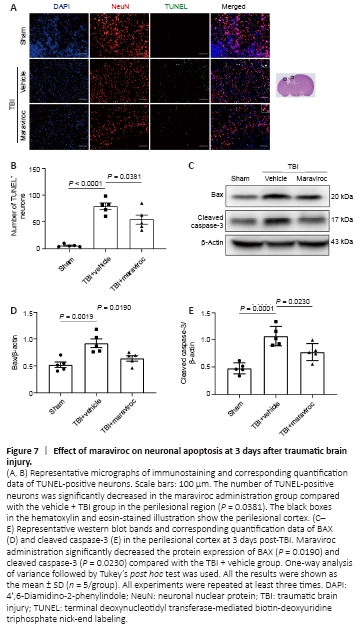
Excessively activated inflammatory responses, NLRP3 inflammasomes, and A1 astrocytes are closely related to the prevalence of apoptosis (Liu et al., 2013; Roth et al., 2014; Liddelow et al., 2017; Skelly et al., 2019). We estimated the effect of maraviroc on neural cell death at 3 days post-TBI, and western blotting analyses were performed to quantify apoptotic cells. Maraviroc administration decreased the levels of cleaved caspase-3 and the apoptosis regulator BAX compared with vehicle administration (Figure 7A and B). In addition, double staining with the TUNEL assay and neuronal nuclear protein revealed many more apoptotic cells in the vehicle treatment group than in the sham group, but maraviroc treatment significantly decreased the apoptotic index compared with vehicle treatment (Figure 7C–E).