脑损伤
-
Figure 3|CGA induces neuroprotective effects on the brain tissue after HIBD in newborn mice.
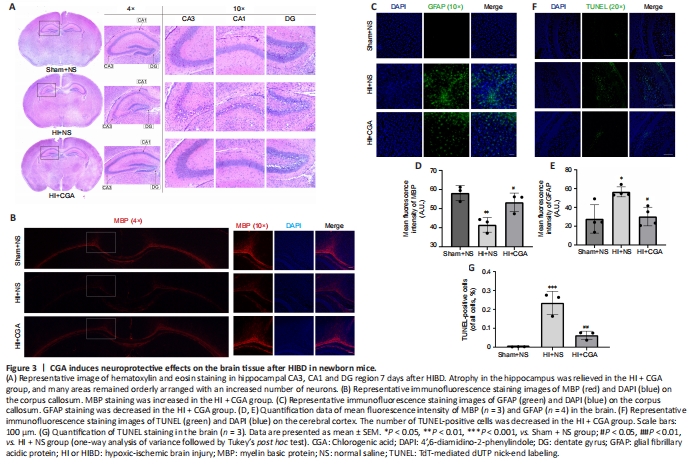
To examine neuronal arrangement after treatment, hematoxylin and eosin staining on brain slices of mice was performed seven days after HIBD (Figure 3A); these slices directly revealed the integrity of the cerebral hemispheres. Brain cells in hippocampal CA3, CA1, and dentate gyrus areas of the Sham + NS group were arranged in a regular pattern. In the HI + NS group, the hippocampal area was atrophied, with apparent cell arrangement disorder, rupture of nucleus, and partial neuron loss. In the HI + CGA group, these changes were alleviated; the atrophy of the hippocampus was relieved, and many areas were orderly arranged with an increased number of neurons.
To investigate whether CGA accelerates axon repair in neonatal HI-injured mice, immunofluorescence staining was used to detect the expression of MBP and GFAP. MBP staining only detects the corpus callosum part in the brain, because this region highly expresses MBP. MBP immunopositivity on the corpus callosum of the HI + NS group was significantly lower than that of the Sham + NS group (P = 0.0076); in the group treated with CGA, upregulated MBP expression was observed. GFAP expression was significantly upregulated in the HI + NS group, whereas the group treated with CGA showed inhibited GFAP expression (P = 0.0189; Figure 3B–E). Furthermore, a significant increase in the number of TUNEL-positive cells after HI injury was observed, and this increase was reduced by CGA intervention (P = 0.0034; Figure 3F–G). Together, these results indicate that CGA promotes axon repair and reduces the number of TUNEL-positive cells with neuroprotective effects on newborn mice after HIBD.Figure 6|CGA protects primary cortical neurons from OGD-induced damage.

We next examined the effects of various concentrations CGA on the viability of primary cortical neurons using CCK-8 assay. Treatment of neurons with CGA at concentrations up to 100 μM for 24 hours did not induce any toxic effects; decreased neuronal viability was observed at a dose of 120 μM (P = 0.0401; Figure 6A). Next, neurons were pretreated with different concentrations of CGA (0–120 μM) and then subjected to OGD injury. The therapeutic effect of CGA on primary cortical neurons treated with OGD was dose-dependent (P < 0.0001; Figure 6B). From these results, we selected the concentration of 100 μM CGA for subsequent experiments.
After subjecting primary cortical neurons to OGD for 24 hours (Xu et al., 2020) to simulate the hypoxic-ischemic environment, analysis of cells using an optical microscope showed that most axons were broken following OGD exposure. Notably, treatment with CGA alleviated the effects of OGD. CGA also reduced the production of reactive oxygen species (Figure 6C and D). Results of immunofluorescence staining of GPX4 (P = 0.0103), the neuronal markers NeuN (P = 0.0436) and MAP2 (P = 0.0481), and TUNEL-positive cells (P = 0.001) on primary cortical neurons 24 hours after exposure to OGD (Figure 6E–L) were consistent with in vivo findings. In addition, the mRNA levels of glutathione (P < 0.0001) and GPX4 (P < 0.0001) were significantly lower in the OGD group than those in the control group, while the mRNA level of cyclooxygenase-2 was higher than that in the control group (P < 0.0001). Notably, CGA partially reversed these mRNA levels (Figure 6M). Together these findings indicate that CGA treatment suppressed OGD-induced damage and reduced the number of TUNEL-positive cells on primary neurons.
Figure 7|Erastin partially reverses the neuroprotective effect of CGA following HIBD in newborn mice.
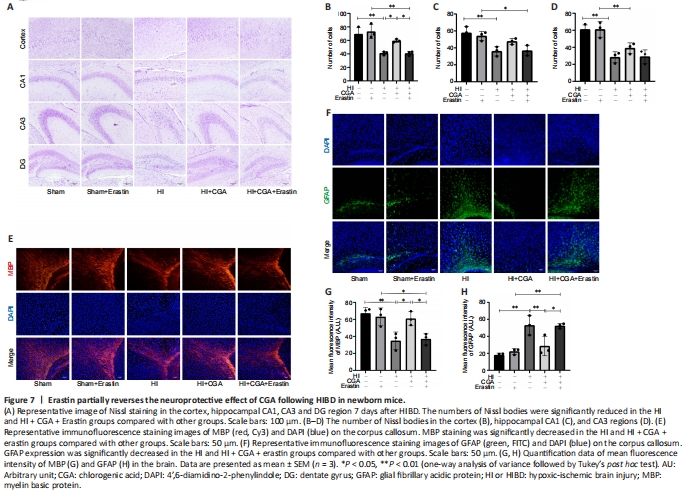
To determine whether the effects of CGA against HIBD were mediated by the system Xc–/GPX4, erastin (a system Xc– inhibitor) was applied. To exclude the effects of erastin on mice, a control + erastin group was established. Changes in brain tissue structure 7 days after HIBD treatment were examined by Nissl staining (Figure 7A–D). In both HI and HI + CGA + erastin groups, the damage was decreased. The number of Nissl bodies in the cortex, hippocampal CA1, CA3 and dentate gyrus areas was markedly decreased following HIBD treatment (P < 0.05). In the sham and sham + erastin groups, the Nissl bodies showed neat and dense arrangement. Immunofluorescence staining of MBP and GFAP is shown in Figure 7E–H. In the sham group, the axons around the corpus callosum appeared dense and branched, while GFAP immunopositivity was low. CGA treatment restored the growth of axons, suppressed the expression of GFAP (P = 0.0047), and increased the number of Nissl bodies. Erastin treatment partially alleviated the changes induced by CGA treatment and partially reversed the neuroprotective effect of CGA treatment.