脑损伤
-
Figure 2|Determination of the observation area as the cortex and hippocampus and successful labeling of pericytes.
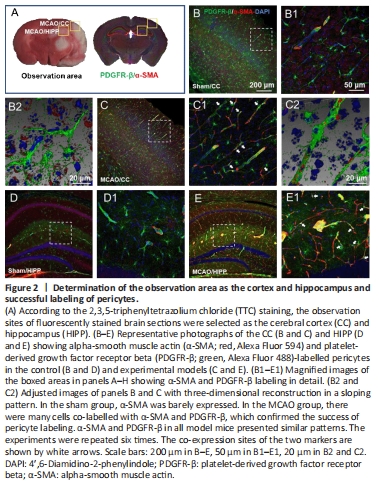
Initially, we planned to focus on the cortex-penetrating arterioles and the branches from the epithelial layer to the middle cortex because in our previous study, the in vivo two-photon imaging study of CBF focused on this area of the brain (Zhang et al., 2020). In view of the locations of cerebral infarction we observed, we decided to examine the cortex and hippocampus (Figure 2A). We selected two markers, α-SMA and PDGFR-β, to identify pericytes. The results showed that α-SMA was barely expressed in normal mouse brain tissues, and that it did not colocalize with PDGFR-β (Figure 2B and C). In the MCAO model mice, the cortex and hippocampus in the ischemic side of the brain tissue generally co-expressed α-SMA and PDGFR-β (Figure 2D and E).
Figure 3|Changes in the expression of the pericyte marker PDGFR-β at different time points after cerebral ischemia/reperfusion.
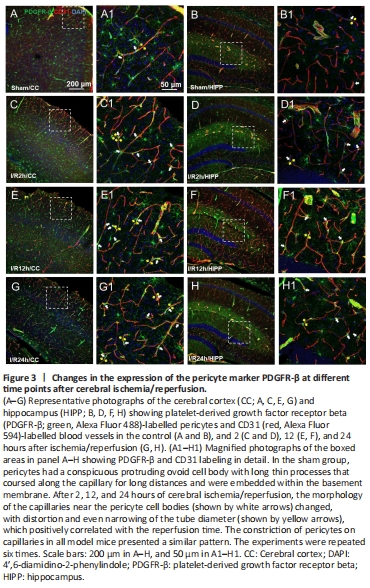
As observed in the CNS, around the capillaries lableled with CD31, the pericytes labelled with PDGFR-β had prominent elliptical cell bodies and elongated protrusions (white arrow, Figure 3A1–H1), which were largely circumferential at the arteriole side of the microvascular bed and at branching points and were distributed more longitudinally in the middle of the capillary bed, compared with the model group. This structure is the typical morphology of pericytes and has been mentioned in most previous studies (Hu et al., 2017; Yang et al., 2017). The number of PDGFR-β-positive cells did not show an increasing trend in relation to the time after reperfusion. We observed that the sham operation group had unconstricted microvessels near the PDGFR-β-positive pericytes and no pathological changes (yellow arrows), such as narrowing of the tube diameter and external force contraction (yellow arrows, Figure 3A, B, A1, and B1). In contrast, in the I/R 2 h, I/R 12 h, and I/R 24 h groups, the microvessels near the PDGFR-β-positive cells were narrowed in many places (Figure 3C–H, and C1–H1) and the microvessels were significantly deformed in the I/R 24 h group.
Figure 4|The expression of the pericyte marker α-SMA at different time points after cerebral ischemia/reperfusion.

Pericytes have been shown to express the contractile proteins needed to regulate blood flow, such as α-SMA. In the sham group, α-SMA expression was barely detected (Figure 4A, B, A1 and B1). In the I/R groups, α-SMA-labelled pericytes demonstrated a mesh-like appearance. The microvessels with α-SMA expression showed narrowing of the vessel diameter in multiple sites. Furthermore, the increased α-SMA expression positively correlated with the I/R time course (Figure 4C–H and C1–H1). Compared with the other groups, in the I/R 24 h group, the α-SMA-positive blood vessels narrowed and deformed the most, and even closed completely.
Figure 5|Three-dimensional reconstruction shows the spatial relationship between PDGFR-β and α-SMA expression and the capillaries.
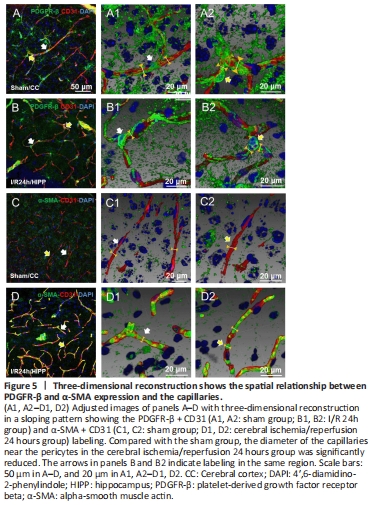
We measured the pericyte location and the diameter of microvessels nearby by fluorescence immunohistochemistry and histochemistry (Figure 5A–D).