脑损伤
-
Figure 2|Severe axonal injury around the hematoma at 24 hours after ICH.
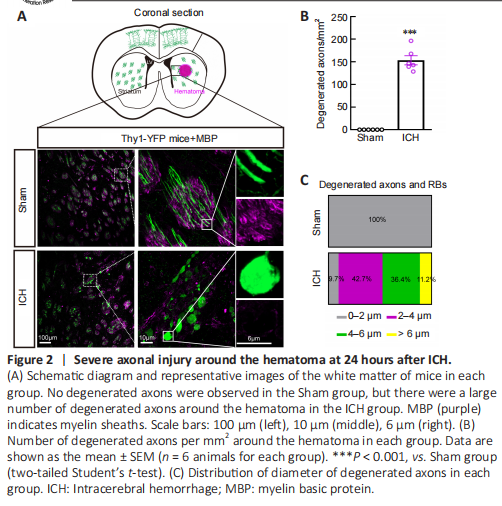
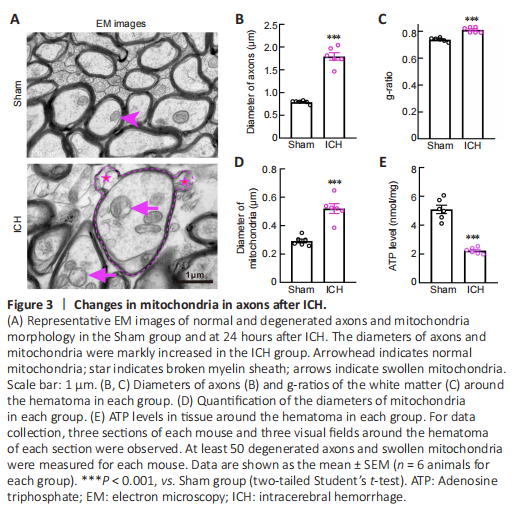
Figure 3|Changes in mitochondria in axons after ICH.
To better visualize morphological changes in the axons after ICH, we used Thy1-YFP mice, in which layer 5 pyramidal cells in the cerebral cortex and their descending axons were labeled with bright green fluorescence (Porrero et al., 2010; Guo et al., 2015). Consistent with our previous results (Yang et al., 2022), we found a large number of degenerated axons around the hematoma, and some axons degenerated into retraction bulbs (RBs) that had round or oval tips at 24 hours after ICH (Figure 2A). In addition, the diameters of myelin were increased and myelin sheaths (detected by staining for myelin basic protein) were drastically compressed (Figure 2A). A total of 153.6 ± 10.1 degenerated axons per mm2 were observed around the hematoma in the ICH group (Figure 2B). The diameters of normal axons in the Sham group were less than 2 μm, while the distribution of axonal diameters in the ICH group was 0–2 μm (9.7%), 2–4 μm (42.7%), 4–6 μm (36.4%), and > 6 μm (11.2%) (Figure 2C). Notably, RBs are the typical pathomorphological feature of degenerated axons after ICH. Moreover, the bright YFP fluorescence made the diameters of normal and degenerated axons (including RBs) appear much larger than the real diameters in electron microscopy images (Figure 3A).
Electron microscopy images showed that degenerated axons were marked by significantly enlarged diameters (P < 0.001; Figure 3A and B). Compared with axons in the Sham group, axons with hypomyelination showed an increased g-ratio in the ICH group (P < 0.001; Figure 3C). Notably, myelin sheaths around swollen axons remained relatively intact (Figure 3A). However, similar to the myelin basic protein staining in Figure 2A, we observed thin, or partially broken, myelin sheaths around the swollen axons (Figure 3A). These results were quite different from the unconsolidated status of myelin sheaths observed several days after ICH (Xia et al., 2019; Yang et al., 2020). These phenomena suggested that axons were more vulnerable than their myelin sheath at the acute phase after ICH.
To examine the morphology and function of mitochondria in the Sham and ICH groups, we first measured the mitochondrial diameter using coronal electron microscopy sections and found that the diameter was significantly increased in the ICH group (P < 0.001; Figure 3A and D). We also collected tissues around the hematoma to assess the ATP level and found that the ATP level in the ICH group was significantly lower than that in the Sham group (P < 0.001; Figure 3E).
Figure 4|Mitochondria impairment in PC12 cells after OH treatment.
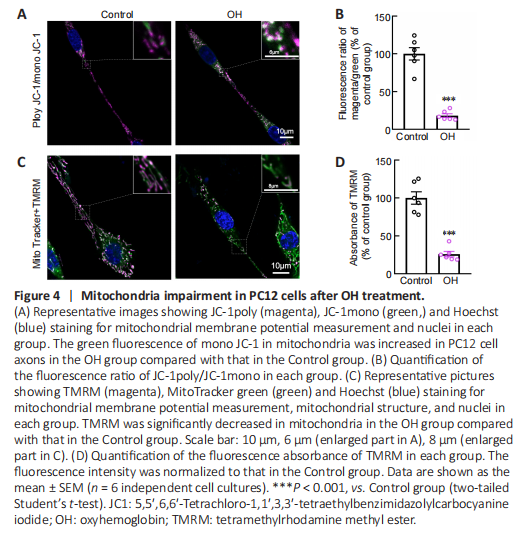
Our previous study showed that OH, a neurotoxic hemolysate after ICH, caused axonal degeneration of primary cultured cortical neurons (Yang et al., 2018b). Therefore, to mimic the ICH model, we injected OH into the striatum. Axonal injury was found around the injection area (Additional Figure 1A and B). To investigate the underlying mechanisms of how mitochondria might be involved in the acute phase of ICH, we used OH to treat PC12 cells. ΔΨm is a key indicator of mitochondrial activity because it reflects the processes of electron transport and oxidative phosphorylation, the driving forces behind ATP production (Zorova et al., 2018). We performed live-imaging of ΔΨm using TMRM and JC-1. We found that JC-1mono was increased and the JC-1poly/JC-1mono fluorescence ratio was significantly decreased in PC12 cell axons in the OH group (P < 0.001; Figure 4A and B). Moreover, the fluorescence density and absorbance of TMRM in the OH group were significantly lower than those in the Control group (P < 0.001; Figure 4C and D). The JC-1 and TMRM results indicated a marked decrease in ΔΨm in PC12 cells after OH treatment.
Figure 5|CypD upregulation in degenerated axons.
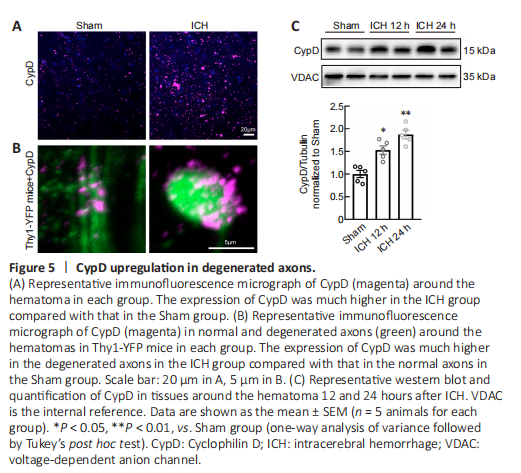
CypD is the mitochondrial cyclophilin isoform in the peptidylprolyl cis-trans isomerase cyclophilin chaperone family. Recent studies have demonstrated that CypD is the essential component of mPTP and regulates the mPTP opening (Kroemer et al., 2007; Bock and Tait, 2020). Overexpression of CypD mediates mitochondrial dysfunction and promotes the opening of the mPTP in various neurological diseases (Schinzel et al., 2005; Sullivan et al., 2005; Du et al., 2008; Warne et al., 2016; Chen et al., 2018b). Our immunofluorescent staining and western blot results showed that the expression of CypD was increased around the hematoma at 12 and 24 hours after ICH (12 hours: P < 0.05, 24 hours: P < 0.01; F(2, 12) = 24.37; Figure 5A–C). Using Thy1-YFP mice, we found that CypD was upregulated especially in degenerated axons (Figure 5B).
Figure 6|CypD deficiency and CsA treatment protect against OH-induced mitochondrial injury in vitro.
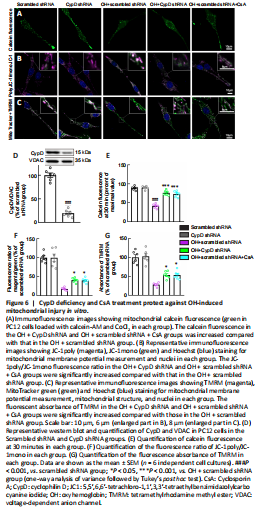
We next used calcein-AM to assess mPTP opening in PC12 cells. Calcein fluorescence was significantly decreased in the OH + scrambled shRNA group compared with that in the scrambled shRNA group (P < 0.001, F(4, 25) = 61.82; Figure 6A and E). Notably, the calcein fluorescence in the OH + CypD shRNA and OH + scrambled shRNA + CsA groups was increased compared with that in the OH + scrambled shRNA group (P < 0.001; Figure 6A and E). These results proved the opening of mPTP after OH treatment and further showed that the mPTP opening could be reversed by CypD knockdown or CsA treatment.
The collapse of the ΔΨm is an early event of mitochondrial dysfunction and leads to the opening of the mPTP (Springer et al., 2018). TMRM and JC-1 were used to measure ΔΨm. We found that the JC-1poly/JC-1mono fluorescence ratio (P < 0.05, F(4, 25) = 51.59) and the fluorescent absorbance of TMRM (P < 0.05, F(4, 25) = 37.79) in the OH + CypD shRNA and OH + scrambled shRNA + CsA groups were significantly increased compared with those in the OH + scrambled shRNA group (Figure 6B, C, F and G). Indeed, the expression of CypD was significantly decreased at 72 hours after lentiviral transfection of CypD shRNA (P < 0.001; Figure 6D). These results suggest that mPTP inhibition caused by CypD knockdown or CsA treatment could protect against decreased mitochondrial ΔΨm in PC12 cells after OH intervention.
Figure 7|Effects of CypD deficiency and CsA treatment on axonal injury after ICH.
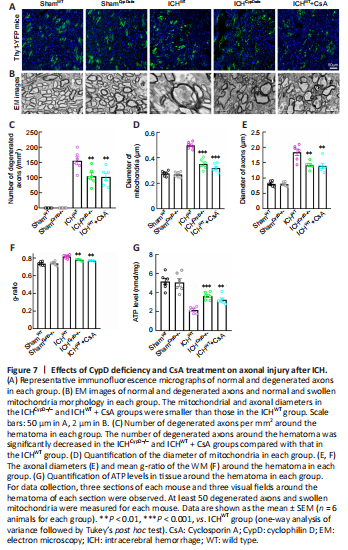
We crossed Thy1-YFP with CypD–/– lines to generate Thy1-YFP::CypD–/– mice. Thy1-YFP::CypD–/– mice, Thy1-YFP::CypD+/– mice, and Thy1-YFP::WT mice treated with the CypD inhibitor CsA were used to assess the involvement of CypD and mPTP opening in axonal degeneration after ICH. Notably, the number of degenerated axons (F(4, 25) = 64.73; Figure 7A–C) around the hematoma (F(4, 25) = 89.58; Additional Figure 1A and B) was significantly decreased in the ICHCypD–/–, ICHCypD+/–, and ICHWT + CsA groups compared with that in the ICHWT group. We next used electron microscopy to investigate the pathomorphological changes in axons and mitochondria. We found that the membrane structure of mitochondria was more integrated in the ICHCypD–/– and ICHWT + CsA groups (Figure 7B). The mitochondrial (P < 0.001, F(4, 25) = 58.50; Figure 7D) and axonal diameters (P < 0.01, F(4, 25) = 42.89; Figure 7E) in the ICHCypD–/– and ICHWT + CsA groups were smaller than those in the ICHWT group. The WM in the ICHCypD–/– and ICHWT + CsA groups showed a decreased g-ratio compared with that in the ICHWT group (P < 0.01, F(4, 25) = 21.26; Figure 7F). In addition, the ATP level around the hematoma was significantly increased in the ICHCypD–/– (P < 0.001, F(4, 25) = 22.12) and ICHWT + CsA (P < 0.01; Figure 7G) groups compared with that in the ICHWT group, suggesting that inhibiting the opening of the mPTP led to significant alleviation of axonal swelling and degeneration after ICH.
Figure 8|CypD deficiency and CsA treatment protect the CST and alleviate motor dysfunction after ICH.
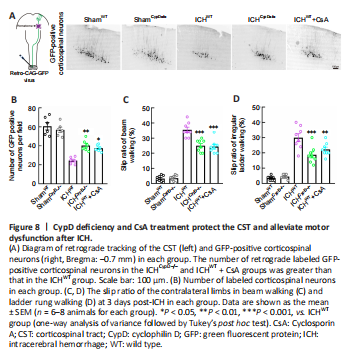 Motor outcome after stroke is dependent on the integrity of the CST (Jang, 2010). To assess the integrity of the CST and CST-associated fine motor function after ICH, viral retrograde tracking (AAV2/Retro-CAG-GFP) from the lumbar spinal cord and fine motor behavioral tests were performed. The number of retrogradely labeled GFP-positive corticospinal neurons (Bregma: –0.7 mm) in the ICHCypD–/– (P < 0.01, F(4, 25) = 32.73) and ICHWT + CsA (P < 0.05; Figure 8A and B) groups was greater than that in the ICHWT group. The slip rates in the beam walking (F(4, 35) = 121.6) and irregular ladder walking tests
Motor outcome after stroke is dependent on the integrity of the CST (Jang, 2010). To assess the integrity of the CST and CST-associated fine motor function after ICH, viral retrograde tracking (AAV2/Retro-CAG-GFP) from the lumbar spinal cord and fine motor behavioral tests were performed. The number of retrogradely labeled GFP-positive corticospinal neurons (Bregma: –0.7 mm) in the ICHCypD–/– (P < 0.01, F(4, 25) = 32.73) and ICHWT + CsA (P < 0.05; Figure 8A and B) groups was greater than that in the ICHWT group. The slip rates in the beam walking (F(4, 35) = 121.6) and irregular ladder walking tests
(F(4, 35) = 48.97) were decreased in the ICHCypD–/– and ICHWT + CsA groups at 3 days after ICH compared with those in the ICHWT group (Figure 8C and D). These results indicate that inhibition of CypD facilitated the integrity of the CST and promoted functional recovery after ICH.