脊髓损伤
-
Figure 1|EPO promotes hindlimb function recovery and tissue repair after SCI.
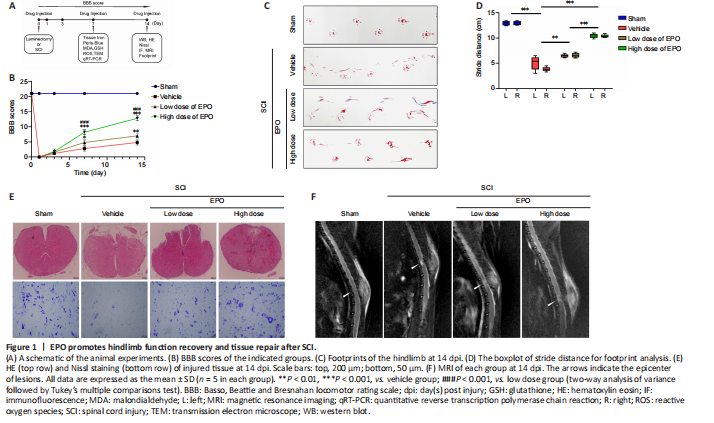
To investigate whether EPO can exhibit a neuroprotective effect in SCI rats, we evaluated the recovery of hindlimb motor function in the four experimental groups using the BBB scale (scored at 1, 3, 7, 14 dpi) and footprint analysis (at 14 dpi). At 1 and 3 dpi, there was no significant difference in BBB score in the two EPO groups relative to that in the vehicle group (low dose vs. vehicle: P = 0.608; high dose vs. vehicle: P = 0.534; Figure 1B). However, from day 7, the BBB scores in the EPO groups were significantly higher than those of the vehicle group (low dose vs. vehicle: P = 0.022; high dose vs. vehicle: P < 0.001). Footprint analysis showed that the stride distance of the EPO groups was remarkably improved, and that the bilateral strides of the EPO groups were more symmetrical than those of the vehicle group (low dose vs. vehicle: P = 0.005 for left and P < 0.001 for right; high dose vs. vehicle: P < 0.001 for both sides; Figure 1C and D). The higher BBB score and longer stride distance in the high dose group relative to those in the low dose group indicated that high dose EPO had a better neuroprotective effect on SCI than the low dose (P < 0.001).
Histology and neuroimaging were used to evaluate the regeneration of injured spinal cord at 14 dpi. HE staining was performed to observe the tissue damage and repair, and Nissl staining was used to evaluate the loss of spinal neurons. In the high dose group, both tissue morphology and Nissl positive cell number were similar to those in the sham group (Figure 1E). MRI scans were performed to evaluate the lesion areas within the spinal cord. Fat-suppressed T2-weighted sagittal MRI showed that the hyperintense area of the lesion in both low and high dose groups were smaller than that in the vehicle group (Figure 1F).
Figure 2|EPO attenuates iron overload, ROS accumulation and MDA content in the injured spinal cord.
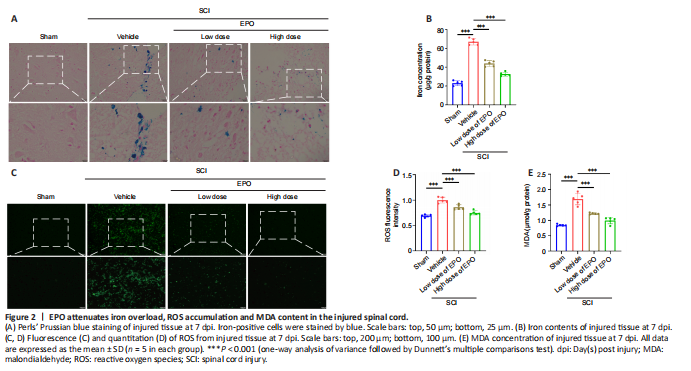
After contusion, subdural bleeding results in accumulation of erythrocytes and hemoglobin around the injured region of the spinal cord, which causes abnormal iron deposition. Perls’ Prussian blue staining showed that the number of blue-stained cells markedly increased in the vehicle group after SCI relative to that in the control group; however, there were fewer blue-stained cells in the low and high dose EPO groups (Figure 2A). Moreover, while the vehicle group had a much higher level of tissue iron concentration relative to the sham group, the iron concentration decreased with the treatment of EPO (P < 0.001; Figure 2B).
In ferroptosis, iron overload generates highly reactive hydroxyl radicals through the Fenton reaction, which results in the accumulation of ROS and lipid peroxidation (Jing et al., 2021). MDA is a natural product of lipid oxidation in living organisms, reflecting the level of lipid peroxidation. We next evaluated ROS and MDA contents respectively. Fluorescence analysis revealed that the ROS fluorescence intensity in the vehicle group had increased after injury relative to those in the sham group (P < 0.001); and the ROS fluorescence intensity was significantly decreased in both the low dose and high dose groups relative to that in the vehicle group (low dose vs. vehicle: P < 0.001; high dose vs. vehicle: P < 0.001; Figure 2C and D). Similarly, MDA content was increased after SCI and diminished by EPO treatment (vehicle vs. sham: P < 0.001; low dose vs. vehicle: P < 0.001; high dose vs. vehicle: P < 0.001; Figure 2E).
Figure 4|The effects of EPO in SCI are abolished by the ferroptosis activator RSL3.
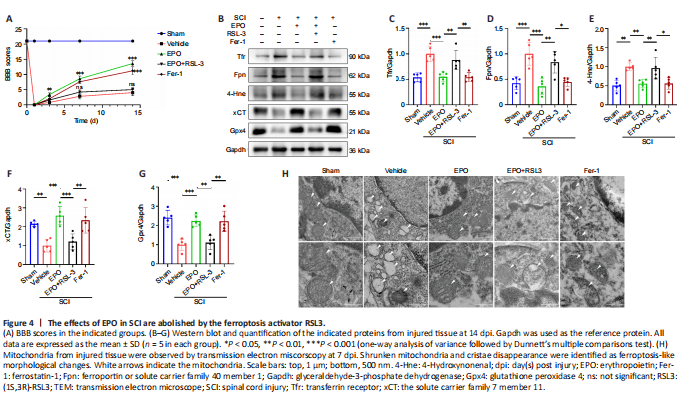
RSL3 is a ferroptosis activator targeting Gpx4 (Shin et al., 2018), and Fer-1 is a ferroptosis inhibitor (Liu et al., 2020b). We evaluated neuronal function recovery and ferroptosis level in SCI rats treated with EPO, EPO + RLS3 and Fer-1. We further determined the expression of xCT and Gpx4 in spinal neurons using immunofluorescence test. A previous study demonstrated that the Fer-1 ferroptosis inhibitor improved the hindlimb motor function of rats after SCI (Ge et al., 2021). We found that rats treated with EPO achieved similar BBB scores as those treated with Fer-1; both scores were significantly higher than the vehicle group (P < 0.001; Figure 4A). The BBB score of the EPO + RSL3 group was significantly decreased relative to that of the EPO group (P < 0.001) and not significantly different from the vehicle group at 14 dpi (P = 0.151).
We next analyzed the expressions of ferroptosis-related proteins. The expressions of Tfr, Fpn and 4-Hne in the EPO and Fer-1 groups were significantly lower than those in the vehicle group, while the expression in the EPO + RSL3 group was increased relative to that in the EPO and Fer-1 groups (EPO vs. vehicle: P < 0.001 for Tfr and Fpn, P = 0.005 for 4-Hne; EPO vs. EPO + RSL3: P = 0.005 for Tfr, P = 0.002 for Fpn, P = 0.01 for 4-Hne; Fer-1 vs. vehicle: P < 0.001 for Tfr and Fpn, P = 0.005 for 4-Hne; Fer-1 vs. EPO + RSL3: P = 0.004 for Tfr, P = 0.014 for Fpn, P = 0.011 for 4-Hne; Figure 4B–E). The expressions of xCT and Gpx4 in the EPO and Fer-1 groups were significantly higher than those in the vehicle and EPO + RSL3 groups, while the expressions in the EPO + RSL3 group were lower relative to those in the EPO and Fer-1 groups (EPO vs. vehicle: P < 0.001 for xCT and Gpx4; EPO vs. EPO + RSL3: P = 0.001 for xCT and Gpx4; Fer-1 vs. vehicle: P = 0.001 for xCT and Gpx4; Fer-1 vs. EPO + RSL3: P = 0.006 for xCT, P = 0.002 for Gpx4; Figure 4B, F–G).
To further confirm the putative role of EPO on SCI-induced ferroptosis, we used TEM to observe the mitochondrial morphology. Ferroptosis-like morphological changes, characterized by the reduction or vanishing of mitochondria crista and outer mitochondrial membrane rupture (Liu et al., 2020a), were observed in the vehicle group and the EPO + RSL3 group (Figure 4H). The mitochondria in the EPO and Fer-1 groups were morphologically closer to those of the sham group.
Figure 5|EPO inhibits neuronal ferroptosis via upregulating expressions of xCT and Gpx4.
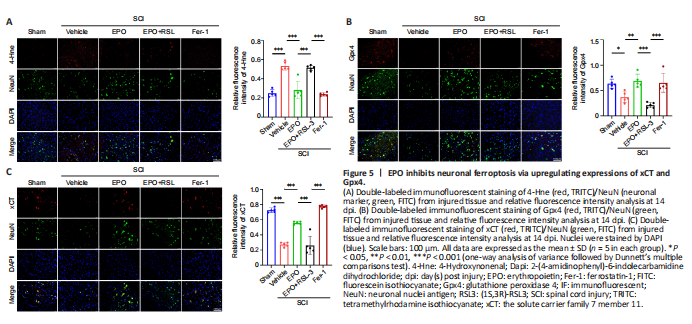
Our results indicated that EPO attenuated ferroptosis within the local tissue after SCI, but whether EPO exerted its effects via inhibiting neuronal ferroptosis was unclear. Therefore, double-label immunofluorescence of spinal cord sections was used to determine the expression of xCT, Gpx4 and 4-Hne in spinal neurons. There were more cells positive for NeuN (a neuronal marker) in the EPO and Fer-1 groups than the vehicle and EPO + RSL3 groups (Figure 5). Furthermore, the fluorescence intensities of xCT and Gpx4 were higher in the EPO and Fer-1 groups relative to that in the vehicle group (xCT: P < 0.001 for both EPO and Fer-1; Gpx4: P = 0.004 for EPO, P = 0.011 for Fer-1), while 4-Hne showed the opposite trend. There were no significant differences in the staining for xCT, Gpx4 and 4-Hne in the EPO + RSL3 and vehicle groups (xCT: P = 0.998; Gpx4: P = 0.273; 4-Hne: P = 0.974). Collectively, our results showed that the therapeutic effect of EPO on SCI rats was related to ferroptosis inhibition which was mediated by upregulating xCT and Gpx4.
Figure 6|EPO alleviates RSL3-induced ferroptosis in PC12 cells.
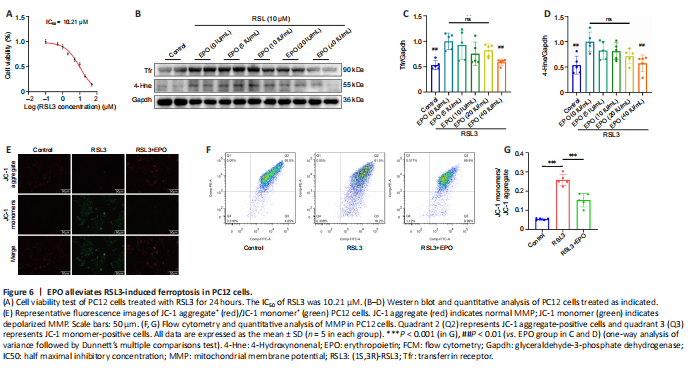
To more closely examine the effects of EPO on SCI-induced ferroptosis in rats, we performed in vitro experiments in PC12 cells. Cell viability assays of RSL3 treatment in PC12 cells were performed, and the IC50 of RSL3 at 24 hours was 10.21 μM (Figure 6A). We then examined the inhibitory effect of EPO at different concentrations (5, 10, 20, 40 IU/mL) against RSL3-induced ferroptosis. EPO at 40 IU/mL showed the most significant suppression on Tfr and 4-Hne (P = 0.006 and P = 0.003 respectively, relative to that at 0 IU/mL; Figure 6B–D). Therefore, RSL3 at 10 μM and EPO at 40 IU/mL were selected to establish the RSL3 + EPO group for the subsequent assays.
Loss of MMP is a feature of early ferroptosis (Chiu et al., 2009). MMP assay with JC-1 showed that the MMP of the RSL3 group decreased, and after treatment with EPO, the MMP was closer to that of the control group (Figure 6E). Moreover, FCM results showed that the number of JC-1 monomer-positive cells in the RSL3 + EPO group was significantly less than that of the RSL3 group (P < 0.001; Figure 6F and G). These results suggested that EPO may improve mitochondrial state in RSL3-treated PC12 cells.
Figure 7| EPO increases expressions of xCT and Gpx4 against RSL3-induced ferroptosis in PC12 cells.
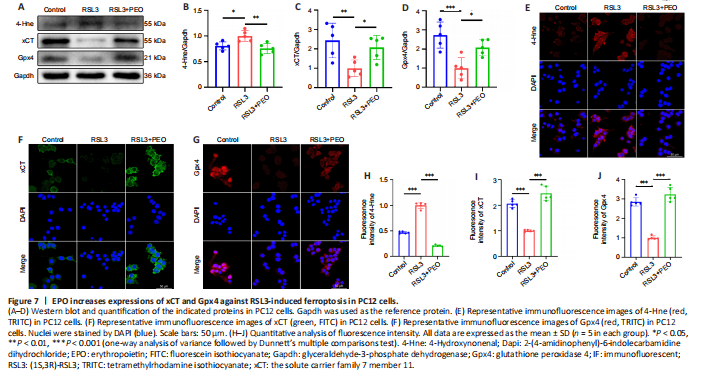
As shown in Figure 7A–D, there was a significant reduction of 4-Hne and a significant increase of xCT and Gpx4 in the RSL3 + EPO group relative to the levels in the RSL3 group (4-Hne: P = 0.003; xCT: P = 0.048; Gpx4: P = 0.019). Immunofluorescence staining revealed stronger fluorescence intensity of xCT and Gpx4 and weaker 4-Hne in the RSL3 + EPO group relative to the RSL3 group (Figure 7E–J). These results suggested that EPO protected PC12 cells from RSL3-induced ferroptosis via upregulating xCT and Gpx4.