脊髓损伤
-
Figure 1|Diagram of experiments in vitro and in vivo.

Approximately 1 × 105 NSCs per well (Control group) were cultured with differentiation medium (DMEM/F12 medium [PM150312, Procell, Wuhan, Hubei, China] supplemented with N2 supplement [17502048, Gibco, New York, NY, USA] and 10% fetal bovine serum [164210, Procell]) for 7 days. NSCs in the OFS group, LV-Tal1 group and LV-NC group were transferred into stainless steel cell culture dishes with differentiation medium and were continuously stimulated by OFS for 7 days (Figure 1). The differentiation medium was changed every 2 days.
Figure 4|OFS combined with NSCs transplantation alleviates cavities formation after SCI.
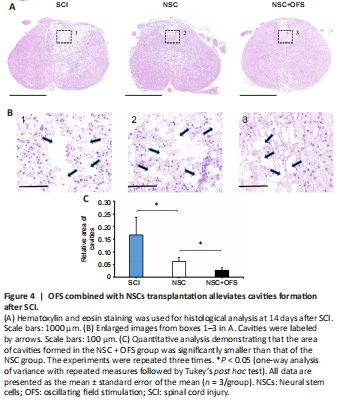
To assess the integrity of spinal cord tissue, H&E staining was performed at 14 days after surgery. Cavities were formed in the epicenter following SCI. The area of cavities formed in NSC + OFS group was significantly smaller than that of the SCI and NSC groups (Figure 4).
Figure 5|OFS promotes the neurogenesis of neural stem cells in vitro.
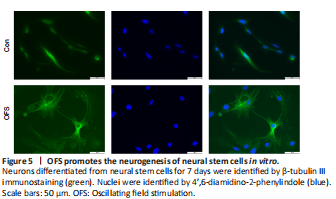
Following the stimulation of differentiation medium for 7 days, all cells were collected. β-Tubulin III IF staining showed that neurons in the OFS group exhibited a more complex, elongated and branched morphology than those in the Control group (Figure 5). WB showed a significant increase of β-tubulin III protein levels in the OFS group compared with those in the Control group (P < 0.05; Figure 6A and B). Additionally, qRT-PCR analysis showed a significant increase of β-tubulin III gene expression in the OFS group compared with that in the Control group (P < 0.05; Figure 6C).
Figure 7|OFS promotes the neurogenesis of transplanted NSCs after spinal cord injury in vivo.
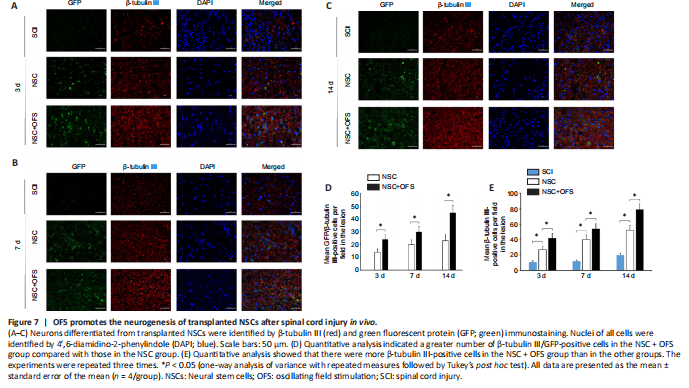
Figure 8|OFS combined with NSCs transplantation promotes axonal regeneration after SCI.
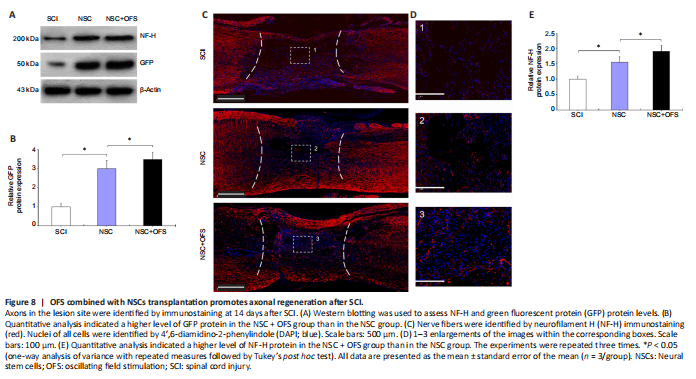
To assess whether OFS regulates the neurogenesis of transplanted NSCs in vivo, IF double staining of GFP and neuron marker β-tubulin III was performed at 3, 7 and 14 days after surgery. The number of GFP- and β-tubulin III-positive cells in the lesion area of the NSC and NSC + OFS groups increased over time, and there were higher numbers of NSC-derived neurons in the NSC + OFS group than in the NSC group at each time point after surgery (P < 0.05; Figure 7). Furthermore, WB results showed that there were higher GFP protein levels in the NSC + OFS group compared with those in the NSC group (P < 0.05; Figure 8A and B), which suggested that OFS treatment is beneficial to the vitality of transplanted NSCs.
To assess the regeneration of axons, IF staining of neurofilament marker NF-H was performed at 14 days post-surgery. In the SCI and NSC groups, there were few NF-H-positive axonal fibers in the lesion site, and these axonal fibers did not grow into the cavities formed after SCI (Figure 8C and D). However, NF-H-positive axonal fibers in the NSC + OFS group were widely distributed in the visual field and penetrated into the lesion (Figure 8C and D). In addition, WB showed that the expression of NF-H protein level was significantly higher in the NSC + OFS group compared with that in the SCI and NSC groups (P < 0.05; Figure 8A and E).