脑损伤
-
Figure 4|WFA improves the pathological changes and decreases the neurobehavioral deficits in the late stage of ICH in vivo.
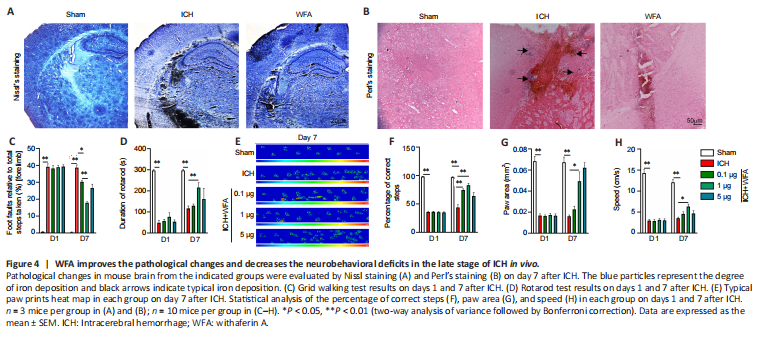
To verify the anti-oxidative damage effects of WFA, we assessed the pathological changes in the perihematomal region after ICH. Nissl staining was conducted to detect the surviving neural cells around the hematoma. As shown in Figure 4A, the amount of Nissl+ cells in perihematomal brain tissue were higher in the WFA group than that in the ICH group. We also examined iron accumulation in the brain perihematomal region by Perl’s staining (Figure 4B). More iron accumulation was found in the perihematomal region of ICH mice than in sham mice. In contrast, treatment with WFA resulted in smaller accumulation of iron compared with the ICH group.
Inhibition of oxidative stress has a beneficial effect on neurofunction recovery after ICH. Thus, to evaluate the effect of WFA treatment on ICH model mice, neurobehavior tests were performed on day 1 and day 7. As assessed by the foot-fault test for forepaw placing and grasping, serious behavioral deficits of the left forepaw were evident in mice at 7 days after ICH (P < 0.0001) (Figure 4C). Notably, animals showed a significant dose-dependent improvement at 7 days after ICH with WFA treatment (ICH vs. ICH + WFA 0.1 μg/kg P = 0.0139, ICH + WFA 0.1 μg/kg vs. ICH + WFA 1 μg/kg, P = 0.0001). In rotarod tests, ICH mice showed a significantly deceased duration of time maintaining balance on the rod (P < 0.0001; Figure 4D). WFA-treated mice (1 μg/kg) exhibited a dramatic increase in the performance of the test (P = 0.0139). Gait impairment was evaluated as the percentage of correct steps, the paw area, and the speed via the Catwalk (Figure 4E–H). As shown in Figure 4F, the percentage of correct steps decreased in the ICH model mice compared with the sham group (P < 0.0001). Treatment with WFA dose-dependently recovered the percentage of correct steps in ICH mice (P = 0.0012, P = 0.0044). Paw area is the area of the paw in contact with road surface during the motion phase. The paw area in the ICH mice was significantly decreased compared with that of the sham mice (P < 0.0001; Figure 4G). However, the paw area of ICH mice dose-dependently increased after treatment with WFA (P = 0.0474). Run speed reflects the crawling ability of mice. As shown in Figure 4H, the run speed in the sham group was faster than that in the ICH group (P < 0.0001), but it was highly recovered after treatment with 1 μg/kg WFA (P = 0.0305). We also observed severe behavioral deficits in animals 1 day after ICH (Figure 4C–H). WFA treatment had no effect on the neurobehavior test results at 1 day post-ICH.
Figure 6|WFA upregulates nuclear factor E2-related factor 2 (Nrf2) expression and promotes nuclear translocation of Nrf2 after ICH in vivo and in vitro.

To explore the mechanisms underlying the upregulation of HO-1 expression with WFA treatment after ICH, bioinformatics analysis was used to identify the key target(s) of WTA. The comparative toxicogenomic database (CTD) was used to search for genes interacting with WFA, and a total of 58 genes were retrieved. Next, all these genes including HO-1 were used for subsequent analysis and a total of 15 genes that directly interacted with HO-1 were found using the STRING database. Among these genes, the nuclear factor erythroid 2-related factor 2 (NFE2L2) gene overlapped with genes that interact with WFA (Figure 6A). Nrf2 is normally sequestered in the cytoplasm and bound to keap1, and it is dissociated from keap1 and subsequently promotes nuclear translocation of Nrf2 under stress (Imai et al., 2021). Thus, the effects of WFA on Nrf2 nuclear translocation during ICH were evaluated. As shown in Figure 6B and C, while the level of Nrf2 expression showed non-significant changes both in the cytoplasm and nucleus in ICH mice, WFA (1 μg/kg) induced a further reduction of Nrf2 expression in the cytoplasm (P = 0.003) and significant elevation of Nrf2 expression in the nucleus (P = 0.0391); higher Nrf2 expression and predominant Nrf2 nuclear location were observed in response to WFA treatment levels in ICH mice (P < 0.001; Figure 6D).
We further investigated the impact of WFA treatment on Nrf2 in vitro using the hemin-induced cell injury model. As shown in Figure 6E–G, nuclear translocation of Nrf2 increased dose-dependently after WFA treatment for 24 hours in hemin-induced SH-SY5Y neuroblast injury (P = 0.0349). The cytoplasmic fractions of Nrf2 were dose-dependently decreased in hemin-induced SH-SY5Y neuroblast injury after WFA treatment for 24 hours (P = 0.0035). Notably, WFA treatment had no effect on the expression of Nrf2 in the nucleus and cytoplasm in the normal medium (P > 0.05). Similar results were shown in immunofluorescence analysis. The overlays of Nrf2 and DAPI images of the two detection channels were used to generate scatterplots and to calculate the Pearson’s correlation coefficient (Rr) and Manders parameter of the two fluorescent signals (Dunn et al., 2011). Rr values > 0.5 or Manders values > 0.6 indicate co-localization. As shown in Figure 6H–K, WFA treatment promoted Nrf2 nuclear translocation in hemin-induced SH-SY5Y neuroblast injury in a dose-dependent manner. In contrast, Nrf2 expression showed no changes in cells exposed to 10?nM WFA in normal medium. These findings suggest that WFA promoted the translocation of Nrf2 from the cytosol to the nucleus after ICH.
Figure 8|WFA induces HO-1 expression, resulting in ameliorated ferroptosis in hemin-induced SH-SY5Y neuroblast injury in vitro.
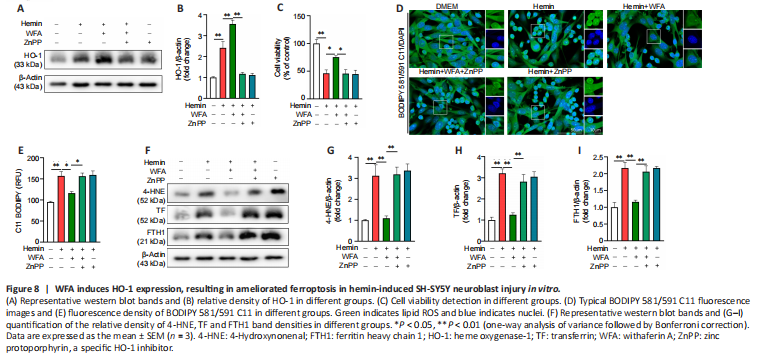
Following ICH, the same series of molecular events is found in oxidative stress and the ferroptosis process, such as lipid peroxidation and ROS (Ren et al., 2021). Therefore, WFA may induce HO-1 expression to attenuate oxidative stress associated with ferroptosis-driven cell death after ICH. In addition, zinc protoporphyrin (ZnPP), a specific HO-1 inhibitor, was used to evaluate the effect of HO-1. HO-1 protein expression after ZnPP treatment is shown in Figure 8A and B, and the results showed that ZnPP significantly down-regulated HO-1 protein level (P < 0.0001). To date, specific ferroptosis markers have not been identified, but several features can separate ferroptotic cell death from other forms of cellular death (Hadian and Stockwell, 2020; Tang et al., 2021). Thus, we first assessed cell viability and found that WFA treatment markedly favored the survival of SH-SY5Y neuroblast in response to hemin-induced injury (P = 0.0484) but was reversed following ZnPP treatment (P = 0.0376; Figure 8C). Next, lipid peroxidation was determined by evaluating lipid ROS and 4-HNE. The level of lipid ROS was assessed by BODIPY 581/591 C11 oxidation. Lipid ROS markedly increased in the hemin-induced cell injury model (P = 0.0010) but was restored following WFA treatment (P = 0.0186). ZnPP abrogated this ferroptosis-associated change (P = 0.0204; Figure 8D and E). We also examined 4-HNE level by western blotting. As shown in Figure 8F and G, the hemin-induced cell injury model exhibited a highly elevated level of 4-HNE protein compared with controls (P = 0.0057). WFA treatment decreased 4-HNE content to the level in the control group (P = 0.0078). However, inhibition of HO-1 with ZnPP significantly reversed it (P = 0.0063). Next, transferrin (TF) and ferritin (FTH1), key proteins of iron metabolism, were detected by western blotting. As shown in Figure 8F, H and I, the protein level of TF and FTH1 was significantly elevated in the hemin-induced cell injury model (P = 0.0002, P = 0.0006) and WFA decreased both TF and FTH1 expression compared with that in the controls (P = 0.0006, P = 0.0019). Furthermore, inhibition of HO-1 with ZnPP significantly increased TF and FTH1 expression (P = 0.0034, P = 0.0044).
Figure 9|WFA combined with ferrostatin-1 influences cell damage in response to hemin-induced cell injury in vitro.

To further confirm the causal link between WFA and ferroptosis, ferrostatin-1 (fer-1), a specific ferroptosis inhibitor was used. Cell viability significantly decreased (P < 0.0001) and the LDH release significantly increased (P = 0.0002) in SH-SY5Y neuroblasts under injury condition. WFA treatment significantly increased SH-SY5Y neuroblast viability (P = 0.032) and reduced LDH release to a larger extent (P = 0.0026) than that with hemin alone. Moreover, this neuroprotective effect was further enhanced by ferroptosis inhibition following treatment with WFA combined with fer-1 (P = 0.0055, P = 0.0005; Figure 9A and B). In addition, the level of lipid ROS markedly increased in hemin-induced SH-SY5Y neuroblast ferroptotic cell death (P < 0.0001). The expression of 4-HNE was significantly enhanced after ICH (P = 0.0008). WFA treatment prevented the upregulation of lipid ROS (P = 0.0029) and 4-HNE (P = 0.0430) after ICH. Of note, WFA combined with fer-1 treatment also improved these changes associated with ferroptosis (P = 0.0002, P = 0.0075; Figure 9C–F). We evaluated oxidative damage and the results showed that WFA treatment reduced MDA content (P = 0.0029) and increased GSH-Px activity (P = 0.0133) after ICH. The combination of WFA and fer-1 further increased this trend (P = 0.0009, P = 0.0137; Figure 9G and H).