脊髓损伤
-
Figure 1|PLX3397 attenuates astrocytic scar formation and increases the inflammatory response following SCI.

To assess the potential contribution of microglia to glial scar formation following SCI, we performed a microglial ablation experiment and analyzed its consequences on glial scar formation at 14 days, when the morphology of the astrocytic scar boundary is similar to that at 4 or 8 weeks (Wanner et al., 2013). Immunofluorescence staining showed that in the SCI group, activated astrocytes organized and formed a compact, dense astrocytic scar border. However, when microglia were depleted by PLX3397, GFAP+ astrocytes were disorganized around the lesion center, without any particular direction, and failed to form a dense, compact astrocytic scar (Figure 1A). The lesion size was determined by calculating the area of GFAP-negative staining. PLX3397 administration significantly increased the lesion size compared with that of the SCI group (P < 0.0001; Figure 1D).
Next, we investigated the inflammatory infiltrates at 2 weeks postinjury. Activated microglia and peripheral blood-derived macrophages were detected by CD68 immunoreactivity. In the spinal cord of SCI animals, CD68+ inflammatory cells were well enclosed in the lesion center by a dense glial scar. However, PLX3397 treatment led to a large number of inflammatory cells spreading into the vicinity across the glial border (Figure 1B). Furthermore, the levels of three critical proinflammatory factors (TNF-α, IL-6, and IL-1β) were detected at 14 days after SCI. Microglial depletion by PLX3397 significantly increased the levels of the three proinflammatory factors compared with the levels in SCI mice (all P < 0.05; Figure 1E–G). Exacerbated inflammatory response often leads to neural cell death after CNS injury. To investigate whether microglial elimination contributes to neuronal death, aCasp3/NeuN colabeling was conducted to examine neuronal death at 14 days after SCI. As shown in Figure 1C and H, aCasp3 expression was very low in sham mice, and the percentage of aCasp3+/NeuN+ cells in the PLX3397 group was significantly higher than that in the SCI group (P = 0.007).
Figure 2|Microglial depletion results in a reduced number of proliferating cells (especially astrocytes) in the spinal cord lesion area.
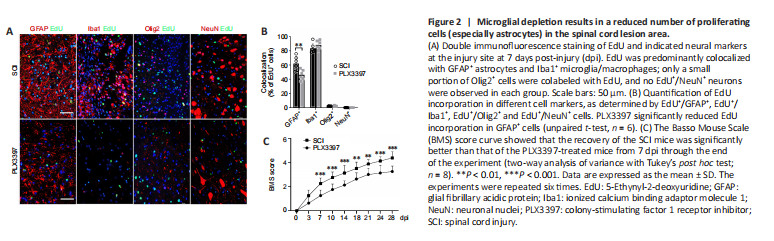
To detect proliferating cells in the lesion area, we intraperitoneally injected EdU (5 mg/kg of body weight) 72 hours after injury to label the newly formed cells at 7 dpi. Microglial elimination significantly decreased the number of EdU+ proliferating cells in the lesion center (Additional Figure 2A and B). Double staining of EdU with GFAP, NeuN, Olig2 and Iba1 was performed to further specify the EdU incorporation in astrocytes, neurons, oligodendrocytes and microglia/macrophages, respectively. EdU was mainly colocalized with GFAP+ signals and Iba1+ signals, and no NeuN+/EdU+ neurons were detected (Figure 2A and B). A marked reduction of GFAP+/EdU+ cells was detected in PLX3397-treated mice compared with the SCI mice, suggesting a reduction in the number of newly proliferated astrocytes.
BMS locomotor rating scores were used to assess the behavioral recovery of mice every 3–4 days for 28 dpi. After SCI, BMS scores in both groups were 0 and then gradually recovered. However, the recovery rate of the SCI group was faster than that of the PLX3397 group (Figure 2C). BMS scores in the PLX3397 group were lower than those in the SCI group, and the difference in scores was statistically significant starting at 7 dpi and continuing throughout the observation period (P < 0.01). At 28 dpi, occasional plantar stepping was observed in SCI mice, but only dorsal stepping was observed in PLX3397-treated mice.
Figure 4|Microglial depletion inhibits phosphorylated STAT3 (pSTAT3) in astrocytes in spinal cord tissue.
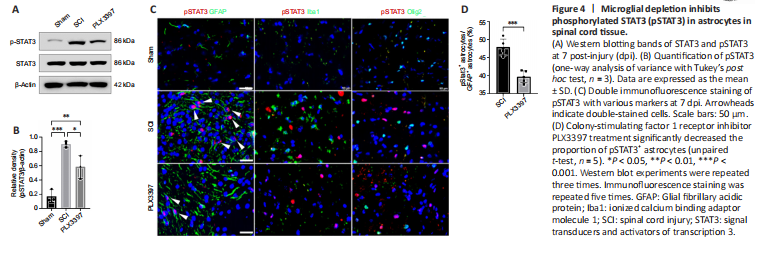
Our western blotting data showed that total STAT3 was expressed similarly between the three groups. Phosphorylated STAT3 levels were significantly increased in mice at 7 dpi compared with those in the sham group (P = 0.0004; Figure 4A and B). PLX3397 administration abolished SCI-induced pSTAT3 upregulation. Double immunolabeling was used to detect pSTAT3 expression in different cells, and the results showed that pSTAT3 signals were predominantly colocalized with GFAP (Figure 4C). No pSTAT3 signal was observed in Iba1+ macrophages/microglia, Olig2+ oligodendrocyte cells or NeuN+ neurons (Figure 4C). Moreover, the percentage of pSTAT3-positive astrocytes in the spinal cord of SCI animals was significantly higher than that in PLX3397-treated animals (P = 0.0002; Figure 4D).
Figure 5|LPS-activated microglia promote astrocyte proliferation in vitro.
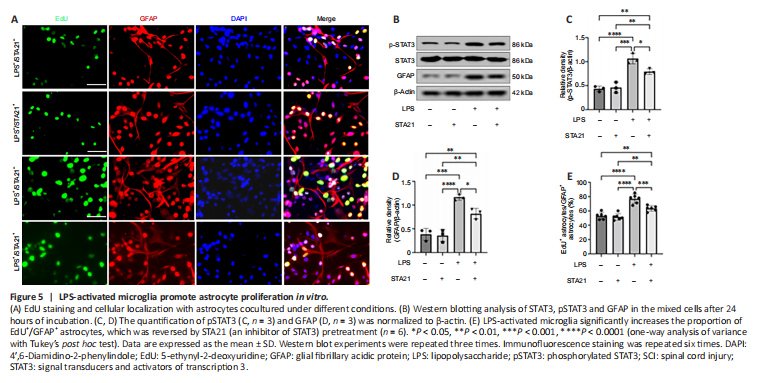
To obtain direct evidence that STAT3 signaling activation mediates crosstalk between microglia and astrocytes, we cocultured microglia and astrocytes in vitro. LPS was used to activate microglia (BV2 cells), and STA21 (a STAT3-selective inhibitor) was used to block STAT3 signaling. Western blotting showed that the GFAP level in astrocytes cocultured with LPS-activated microglia was significantly increased by 3.02-fold compared with that in cells cocultured with nonactivated microglia; however, when astrocytes were pretreated with STA21, the activated microglia-induced enhancement of GFAP expression was blunted (Figure 5B and D). Interestingly, we found STA21 only inhibited LPS-activated microglia-induced GFAP expression. Similarly, regardless of whether astrocytes were pretreated with STA21, pSTAT3 expression was low when astrocytes were cocultured with microglia without LPS activation. LPS-activated microglia had markedly increased pSTAT3 level compared with nonactivated microglia, and this enhancement was significantly reduced by STA21 (Figure 5B and C). Next, we examined astrocyte proliferation by double staining with GFAP and EdU, and the results showed that coculture with LPS-activated microglia significantly increased the number of proliferating astrocytes compared with that in the group cocultured with nonactivated microglia. These enhancement effects were inhibited by STA21 treatment (Figure 5A and E). These results indicated that activated microglia promote astrocyte proliferation by modulating STAT3 signaling.