脊髓损伤
-
Figure 2|G-Rb1 improves the motor function of mice.

To detect the effect of G-Rb1 on the motor function of mice with SCI, we used footprint analysis, rotarod system tests, oblique board tests, and BMS scores to evaluate the motor function of mouse hind limbs. The footprint analysis showed that compared with footprints in the SCI group, footprints in the G-Rb1 group were improved, the step length was longer, and the step width was shorter (Figure 2A–C). The rotarod test found that the exercise time of the G-Rb1 group on the rolling axis was longer than that of the SCI group (Figure 2D). The oblique board test showed that the inclination angle of the inclined plate in the G-Rb1 group was greater than that in the SCI group (Figure 2E). Finally, the activity of the hind limbs of mice in each group was evaluated using the BMS score. The results showed that the movement of hind limbs in the G-Rb1 group was improved (Figure 2F). The above experiments comprehensively demonstrated the improvement in motor function of mice. Furthermore, immunofluorescence results showed that after treatment, anterior horn motoneuronal survival was higher in the G-Rb1 group than in the SCI group, and motoneurons were more morphologically intact (Figure 2G and H).
Figure 3|G-Rb1 inhibits oxidative stress and protects mitochondria after SCI.

After SCI, mitochondria are severely injured, and their energy production is decreased, with increased production of ROS (Slater et al., 2022). G-Rb1 has antioxidant activity in myocardial ischemia (Fan et al., 2020), but whether it protects mitochondria through its antioxidant effects after SCI is unclear. First, a mitochondrial probe was used to detect the number of mitochondria in PC12 cells. The results showed that the number of mitochondria in neurons was increased after treatment (Figure 3A and B). To measure the oxidative stress in each group, ROS, MDA, SOD, and GSH-Px kits were used to detect the content of key substances involved in oxidative stress. The results showed that the level of ROS in neurons in the G-Rb1 group was lower than that in the OGD group (Figure 3C and D). MDA reflects the status of lipid peroxidation (Qi et al., 2014), and its content in neurons was also decreased after treatment (Figure 3E). The content of antioxidant SOD and activity of GSH-Px were increased after treatment (Figure 3F and G). To further verify the antioxidative stress activity of G-Rb1 in vivo, ROS, MDA, SOD, and GSH-Px kits were used to detect oxidative stress at the injury site (Figure 3H–J). Consistent with the in vitro findings, the G-Rb1 group showed decreased contents of ROS and MDA compared with the SCI group. Moreover, the G-Rb1 group exhibited increased SOD contents and GSH-Px activity compared with the SCI group. Therefore, G-Rb1 protected the mitochondria of neurons by inhibiting oxidative stress.
Figure 4|G-Rb1 improves energy deficiency and neuronal apoptosis.
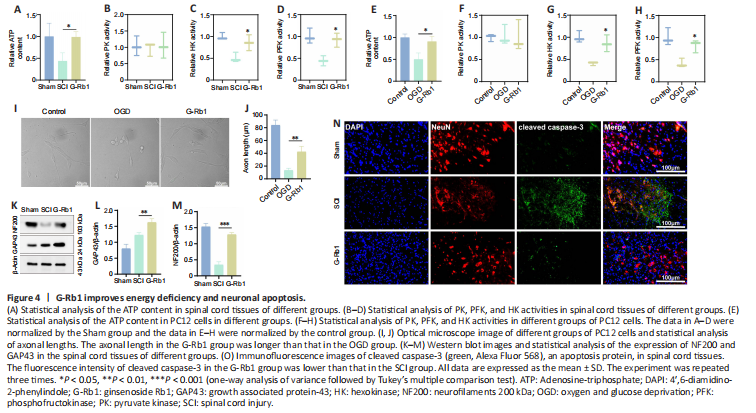
G-Rb1 has been reported to have therapeutic effects on ischemic diseases, such as myocardial ischemia and cerebral ischemia (Slater et al., 2022). Therefore, we considered whether its efficacy is related to the improvement in cell metabolism. First, we collected samples from damaged spinal cord tissue. After G-Rb1 treatment, ATP production in the injured spinal cord was increased (Figure 4A). ATP and lactic acid produced by glycolysis play an important role in neuronal apoptosis and axonal regeneration (Schurr and Passarella, 2022). Therefore, we next detected the activities of key glycolytic enzymes. The results showed that the activities of HK and PFK but not PK were increased by G-Rb1 (Figure 4B–D). Because neurons mediate spinal cord signaling (Ding et al., 2022), PC12 cells were used in all cell experiments. The results were similar to those in animal experiments (Figure 4E–H). Furthermore, using an optical microscope, we found that the axon length of neurons was increased after treatment. Moreover, the cell morphology was improved, and the cell density was increased (Figure 4I). To examine axon regeneration, the protein levels of GAP43 and NF200 were detected, and the western blot results showed that the levels of GAP3 and NF200 in the G-Rb1 group were higher than those in the SCI group (Figure 4K–M). Owing to the improvement in cell morphology and cell density after treatment, we speculate that G-Rb1 is related to the inhibition of apoptosis. Therefore, immunofluorescence staining was performed to detect apoptosis-related proteins, and the results showed that the fluorescence intensity of cleaved caspase-3 in the G-Rb1 group was lower than that in the SCI group in spinal cord tissues. In conclusion, G-Rb1 may reduce neuronal apoptosis by improving energy deficiency (Figure 4N).
Figure 5|Sirt3 is a key factor in the G-Rb1-mediated treatment of SCI.

Because Sirt3 regulates glycolysis and oxidative stress (He et al., 2017; Zheng et al., 2018), we questioned whether G-Rb1 works through Sirt3. First, immunofluorescence was used to detect the expression of Sirt3. The results showed that Sirt3 was expressed in neurons, and the fluorescence intensity in the G-Rb1 group was higher than that in the OGD group (Figure 5A and B). The results of in vivo experiments showed that the protein expression of Sirt3 in the G-Rb1 group was also higher than that in the SCI group (Figure 5C and D). Next, the Sirt3 inhibitor and G-Rb1 were used together. The rotarod test showed that when the Sirt3 inhibitor was used with G-Rb1, the duration of the Sirt3– group on the rotarod was shorter than that of the G-Rb1 group (Figure 5E). Footprint analysis also showed that the step length of the Sirt3- group was shorter, and the step width was longer compared with those in the G-Rb1 group (Figure 5F–H). Additionally, the results of the oblique board test showed that the grasping ability of the Sirt3- group was weaker than that of the G-Rb1 group (Figure 5I). The BMS score revealed that the hind limb motor ability of the Sirt3- group was lower than that of the G-Rb1 group (Figure 5J). Therefore, G-Rb1 promotes the recovery of motor ability in mice, which is related to Sirt3.
Figure 6|Sirt3 is key for G-Rb1 to inhibit oxidative stress in the treatment of SCI.
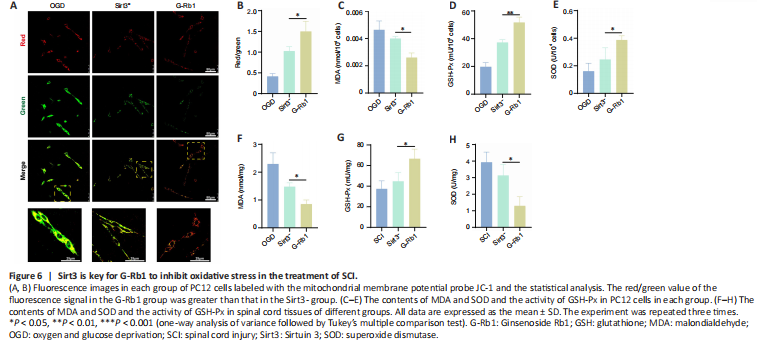
The above experiments demonstrated that G-Rb1 inhibits neuronal oxidative stress and protects neuronal mitochondria. We next questioned whether it works through Sirt3. First, the functional status of mitochondria was detected by JC-1. Compared with the G-Rb1 group, the Sirt3– group showed decreased red/green fluorescence intensity in PC12 cells, suggesting the aggravation of mitochondrial damage (Figure 6A and B). Moreover, the key substances of oxidative stress, such as MDA, SOD, and GSH-Px, in neurons of each group were detected. The results showed that after Sirt3 was inhibited, the MDA content was higher than that in the G-Rb1 group (Figure 6C). In addition, the SOD content and GSH-Px activity were decreased (Figure 6D and E). To confirm these results in vivo, we collected the injured tissues of mice in each group. The results also showed that when Sirt3 was inhibited, the MDA content was increased, and the SOD content and GSH-Px activity were decreased (Figure 6F–H). Therefore, Sirt3 is key for G-Rb1 to inhibit oxidative stress and protect mitochondria.
Figure 7|Sirt3 is involved in the regulation of glycolysis by G-Rb1 in the treatment of SCI.

Because Sirt3 is involved in the regulation of SCI recovery by G-Rb1, we next determined which regulatory processes Sirt3 plays a role in. First, Sirt3-inhibited SCI mice were treated with G-Rb1. ATP detection showed that the intracellular ATP content in the Sirt3- group was lower than that in the G-Rb1 group (Figure 7A). The results of enzyme activity experiments showed that compared with those in the G-Rb1 group, the activities of HK and PFK in the Sirt3- group were increased, but there was no difference in PK activity (Figure 7B–D). To clarify the effect of Sirt3 inhibition on neurons, PC12 cells were used to detect energy metabolism. The results showed that ATP production, HK activity, and PFK activity were decreased after Sirt3 inhibition (Figure 7E–H). To determine whether G-Rb1 inhibits neuronal apoptosis through metabolic reprogramming related to Sirt3, apoptosis-related proteins were detected. The immunofluorescence results showed that after Sirt3 inhibition, the fluorescence intensity of cleaved caspase-3 was higher than that in the G-Rb1 group (Figure 7I–K). In addition, the expression of Bax and cleaved caspase-3 was higher than that in the G-Rb1 group, and the expression of Bcl-2 was lower than that in the G-Rb1 group (Figure 7L–O). In conclusion, Sirt3 inhibition reverses the neuronal metabolic reprogramming and apoptosis caused by G-Rb1.