脊髓损伤
-
Figure 2|Cholesterol-25-hydroxylase (CH25H) colocalization with astrocytes at the lesion site following spinal cord injury (SCI) in a rat model.

Figure 3|Effects of PAR1 inhibition on the astrocyte cholesterol-25-hydroxylase (CH25H) expression following spinal cord injury (SCI) in a rat model.
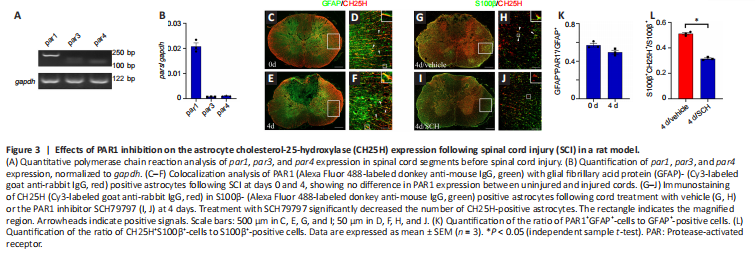
To ascertain whether the astrocytes are involved in thrombin-mediated cholesterol metabolism, immunostaining was performed to detect the cellular distribution of CH25H. The results demonstrated that co-localization of CH25H/GFAP- (Figure 2A–H) and CH25H/S100β-positive astrocytes (Figure 2I–P) was more frequent at 1, 4, and 7 days following SCI in a rat model compared with 0 day (Figure 2Q and R). Because thrombin mediates cell signaling through PAR1, PAR3, and/or PAR4 (Russell, 2003; Wang et al., 2019b), we next examined the expression of these receptors par1 was expressed at higher levels in the cord tissue than par3 and par4 (Figure 3A and B). Immunostaining analysis detected PAR1 in GFAP-positive astrocytes before and after SCI (Figure 3C–F and K), and CH25H expression was significantly decreased in the SCH79797 group compared with that in the control group (Figure 3G–J and L). These findings suggest that CH25H expression in astrocytes is regulated by the thrombin/PAR1 axis in response to SCI.
Figure 4|Cholesterol-25-hydroxylase (CH25H) expression following treatment of astrocytes with thrombin.
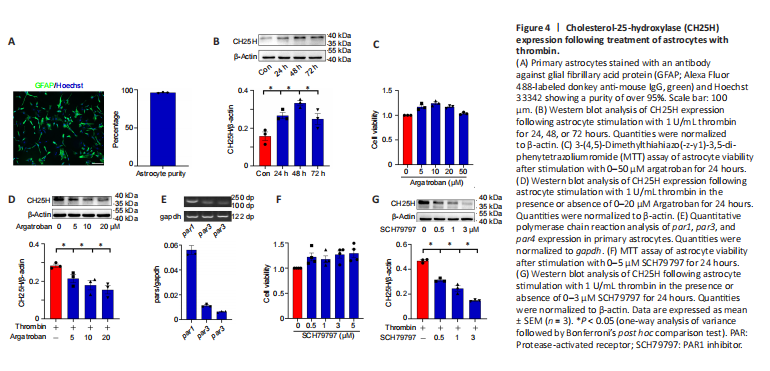
To investigate the role of thrombin in the regulation of CH25H expression by astrocytes, we cultured 95% pure primary astrocytes (Figure 4A). Adding U/mL thrombin to the astrocytes for 24, 48, or 72 hours induced a rapid elevation in CH25H protein levels in the cells (Figure 4B). However, the addition of 5–20 μM of the thrombin inhibitor Argatroban significantly decreased CH25H protein levels, and this effect was not due to cell toxicity (Figure 4C and D). Next, par1, par3, and par4 expression in primary astrocytes was determined by qPCR, and the results were consistent with the in vivo results (Figure 4E). Western blotting showed that CH25H expression was inhibited in a dose-dependent manner by 0.5–3 μM of the PAR1 inhibitor SCH79797 (Figure 4F and G). However, siRNA-mediated knockdown of PAR3 expression did not inhibit the thrombin-induced expression of CH25H, similar to the scrambled siRNA control group (Additional Figure 1A–C). In contrast, treatment with 100 μM (but not 10 μM) of the PAR4 inhibitor tcY-NH2 attenuated CH25H expression (Additional Figure 1D and E). Taken together, these findings indicate that thrombin induces astrocytic expression of CH25H mainly through activation of the PAR1 receptor.
Figure 7|Effects of thrombin-induced production of oxysterol 25-hydroxycholesterol (25-HC) on macrophages.
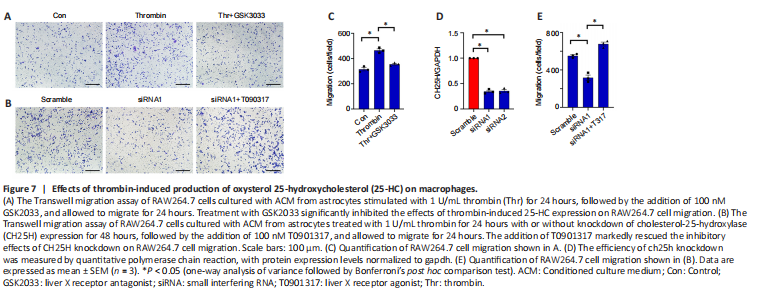
To examine the effects of thrombin-induced 25-HC production on macrophages, we added 0-100 μM 25-HC to the culture medium of RAW264.7 macrophages for 24 hours and observed its effects on cell migration. The Transwell assay results demonstrated that treatment of RAW264.7 cells with 5–100 μM 25-HC markedly increased cell migration compared with a lack of treatment (Additional Figure 2). Next, astrocytes were treated with 1 U/mL thrombin for 24 hours with or without knockdown of CH25H expression for 48 hours, and the ACM was collected to examine its effect on macrophage migration. The Transwell assay results demonstrated that fewer RAW264.7 in the CH25H siRNA group migrated into the lower chamber compared with those in the scrambled siRNA control group (Figure 7B, D, and E). LXRs are endogenous 25-HC ligands that are very important in the regulation of cholesterol metabolism (Zhao et al., 2020). Adding 100 nM of the LXR antagonist GSK2033 to ACM, significantly reduced RAW264.7 migration in the Transwell assasy (Figure 7A and C), suggesting that GSK2033 abrogates the effects of thrombin-mediated astrocyte 25-HC production on RAW264.7 migration. However, adding 100 nM of T0901317, a highly selective LXR agonist, rescued the inhibitory effects of CH25H knockdown (Figure 7B and E). These findings indicate that thrombin-induced astrocyte 25-HC production promotes macrophage migration.
Figure 8|Effects of SCH79797 on the recovery of motor function following spinal cord injury (SCI) in a rat model.
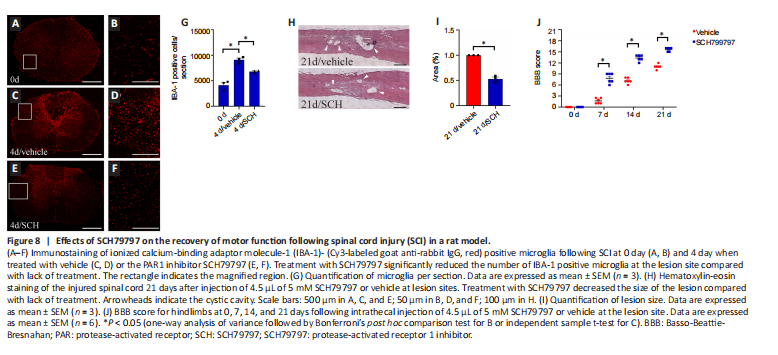
To assess the therapeutic effect of thrombin blockade after SCI, we used BBB motor function scores to assess motor function after SCI. Because thrombin-induced astrocyte 25-HC production is associated with the migration of innate immune cells (Chen et al., 2017; Zhu et al., 2019), we quantified the microglia/macrophages at the lesion site before or after SCI. The number of IBA-1-positive microglia at the lesion 4 days after SCI was significantly increased in comparison with 0 day (Figure 8A–D and G), whereas administration of 4.5 μL of a 5 mM solution of the PAR1 inhibitor SCH79797 reduced the number of IBA-1-positive microglia compared with lack of administration (Figure 8E–G). We further examined the effects of inhibiting microglia migration on the size of the lesion. Observation of hematoxylin-eosin staining sections revealed that the lesioned area of the cord in SCH79797 group was significantly reduced compared with that in the vehicle group at 21 days following contusion (Figure 8H and I).
Next, BBB scores were used to evaluate rat motor function for 21?days after SCI. Compared with a lack of treatment, SCH79797 treatment resulted in higher BBB scores at 7, 14, and 21 days (Figure 8J). These findings indicate that inhibiting thrombin improves rat motor function after SCI.