脑损伤
-
Figure 2|Downregulation of CISD2 in in vivo and in vitro models of CIRI.

To examine the correlation between CISD2 expression and CIRI and oxygen-glucose deprivation and reoxygenation (OGD/R) injury, we established MCAO/R and OGD/R models. In vivo experiments revealed that the degree of behavioral dysfunction and cerebral infarct volume were significantly higher in the I/R group compared with those in the Sham group (all P < 0.01; Figure 2A–C). Moreover, 24 hours after reperfusion, we observed significant downregulation of CISD2 expression in the brain tissue in the I/R group compared with that in the Sham group (P < 0.05; Figure 2D and E). In vitro experiments revealed that the cell survival rates and CISD2 expression were significantly lower in the OGD/R group compared with the control group (all P < 0.01; Figure 2F–I). These results suggest that the expression of CISD2 may be related to neural injury in vivo and in vitro.
Figure 3|Overexpression of CISD2 alleviates I/R injury and inhibits ferroptosis.

To investigate the role of CISD2 in CIRI, we overexpressed or knocked down the gene in mice (Figure 3A–F). Compared with the I/R group, the behavioral function scores indicated that the overexpression of CISD2 alleviated neurological dysfunction 24 hours after reperfusion (P < 0.01; Figure 3G). Compared with the I/R group, the results of 2,3,5-triphenyl tetrazolium chloride staining revealed that the overexpression of CISD2 reduced the cerebral infarct volume (P < 0.0001; Figure 3H and I). However, compared with the I/R group, the knockdown of CISD2 aggravated I/R-induced neurological dysfunction and increased the cerebral infarct volume (all P < 0.05; Figure 3G–I). We evaluated the influence of CISD2 overexpression on neuronal morphology in cerebral tissue via Nissl staining. As shown in Figure 3J, the staining of neurons in the cerebral tissue of mice in the Sham group revealed a large cell volume and abundant cytoplasmic compartments. However, the neurons in the I/R group were sparsely distributed, and the cell bodies were atrophied. This phenomenon was reversed by the overexpression of CISD2. To explore how CISD2 overexpression alleviated CIRI, we examined ferroptosis-related indicators including the expression of GPX4, xCT, TFR1, and the content of GSH, MDA, and Fe2+ in the brain tissue. When compared with the Sham group, the expression of GPX4 and xCT and the content of GSH in the brain tissue were significantly reduced in the I/R group (all P < 0.01; Figure 3K–Q). Furthermore, the content of MDA and Fe2+ as well as the expression of TFR1 in the brain tissue were significantly increased in the I/R
group (all P < 0.01; Figure 3K–Q). When CISD2 was overexpressed, GPX4 and xCT expression, as well as the content of GSH in the brain tissue, were significantly increased compared with the I/R group (all P < 0.05; Figure 3K–Q). Additionally, the contents of MDA and Fe2+, as well as the TFR1 expression in the brain tissue, were significantly reduced (all P < 0.05; Figure 3K–Q) compared with the I/R + AAV-NC group. The transmission electron microscopy indicated that the mitochondrial morphology in the brain tissue from the Sham group was normal, with a complete cristae structure. However, in the I/R group, the mitochondria were shrunken and the crest structures had disappeared. However, when CISD2 was overexpressed, the degree of injury in the mitochondrial morphology was lower (Figure 3R). These data imply that the overexpression of CISD2 alleviated CIRI by inhibiting ferroptosis.
Figure 4|Overexpression of CISD2 alleviates OGD/R injury and inhibits ferroptosis in HT22 cells.
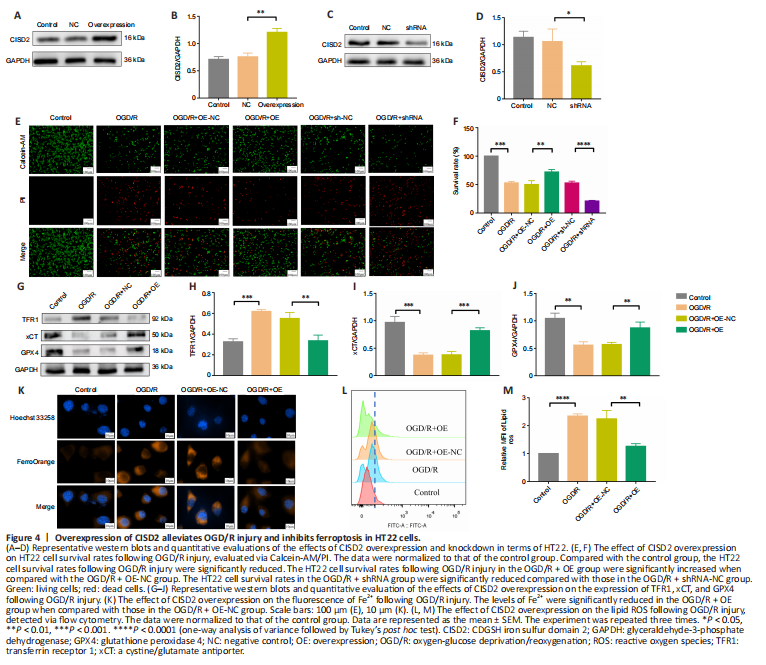
To further explore the neuroprotective effect of CISD2, we overexpressed or knocked down the gene via transfection in HT22 cells (Figure 4A–D). Our investigation of the survival rates indicated that, when compared with the OGD/R group, CISD2 overexpression significantly improved the survival rates of HT22 cells following injury. However, compared with the OGD/R group, the knockdown of CISD2 aggravated the OGD/R injury (all P < 0.01; Figure 4E and F). In addition, the expression levels of GPX4 and xCT were significantly reduced, while the levels of Fe2+ and lipid ROS, as well as the expression of TFR1, were significantly increased in the OGD/R group compared with the control group (all P < 0.01). Moreover, the expression levels of GPX4 and xCT were significantly increased and the levels of Fe2+ and lipid ROS, as well as the expression of TFR1, were significantly reduced in the OGD/R + CISD2-OE group compared with the OGD/R + NC group (all P < 0.01; Figure 4G–M). Together, these results indicate that the overexpression of CISD2 can alleviate OGD/R injury by inhibiting ferroptosis.
Figure 6|ML385 reverses CISD2 overexpression-induced neuroprotective effects and resistance to ferroptosis following CIRI.

We used ML385 to investigate the role of the Nrf2 /HO-1 pathway in the overexpression of CISD2. The behavioral change scores revealed that ML385 reversed the neuroprotective effects of CISD2 overexpression (P < 0.05; Figure 6A), and the volumes of the cerebral infarcts were clearly increased compared with those in the I/R + AAV-CISD2 group (P < 0.0001; Figure 6B and C). Subsequently, we examined the indicators of ferroptosis. When compared with the I/R + AAV-CISD2 group, the expression levels of GPX4 and xCT, as well as the content of GSH in the brain tissue were significantly reduced, whereas the contents of MDA and Fe2+, as well as the expression of TFR1 in the brain tissue were significantly increased in the I/R + AAV-CISD2 + ML385 group (all P < 0.05; Figure 6D–J). The transmission electron microscopy indicated that, compared with the I/R + AAV-CISD2 group, ML385 clearly limited the protective effects of CISD2 overexpression on mitochondrial morphology (Figure 6K). Together, these results suggest that the activation of the Nrf2/HO-1 pathway was involved in the neuroprotective effects and resistance to ferroptosis induced by CISD2 overexpression in mice with CIRI.
Figure 7|ML385 reverses the neuroprotective effects and resistance to ferroptosis in mice with CISD2 overexpression following OGD/R injury.
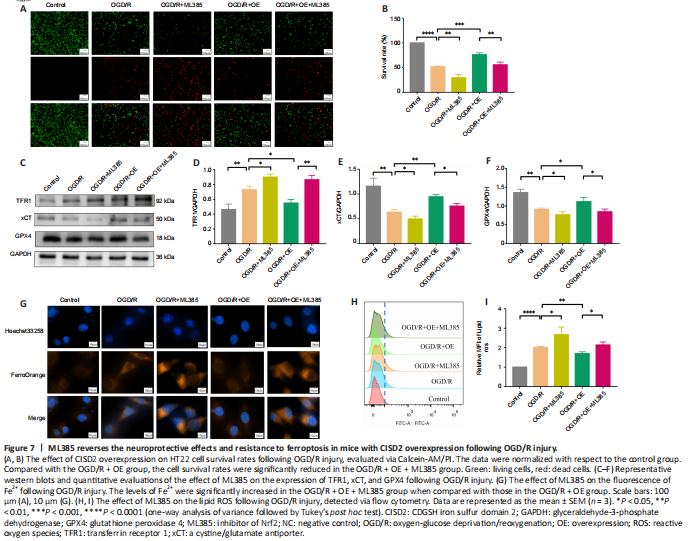
To explore the role of the Nrf2/HO-1 pathway in CISD2-overexpression mediated protective effects, we performed an in vitro experiment. The results revealed that, when compared with the OGD/R + OE group, the cell survival rates were significantly reduced in the OGD/R + OE + ML385 group (P < 0.05; Figure 7A and B). The expression levels of GPX4 and xCT were markedly reduced and the expression of TFR1 was significantly increased after treatment with ML385. Moreover, the inhibition of Fe2+ and lipid ROS via the overexpression of CISD2 was abolished by the application of ML385 (all P < 0.05; Figure 7C–I). Together, these results suggest that activation of the Nrf2/HO-1 signaling pathway may be involved in the protective effects and resistance to ferroptosis induced by the overexpression of CISD2 in HT22 cells with OGD/R injury.