脊髓损伤
-
Figure 1|Aging attenuates neurological functional recovery and tissue repair after SCI.

To assess how aging affects neurological recovery after SCI, three behavioral tests were implemented to analyze the differences between young and aged mice. As presented in Figure 1A and B, the BMS score and subscore were not significantly different between young and aged mice before SCI but trended toward recovery in both young and aged mice after SCI (Figure 1A and B). However, the aged SCI mice had markedly lower scores compared with young SCI mice starting at 7 days post-SCI. The aged group had a higher withdrawal threshold in response to mechanical stimuli post-SCI compared with the young group (Figure 1C). In addition, the aged group exhibited markedly impaired MEP amplitudes and latency periods 56 days post-SCI (Figure 1D–F).
Next, we examined the pathology of the aged spinal cord after SCI. First, we confirmed that the pathological changes 14 days post-SCI were consistent in the young and aged groups (Additional Figure 4) and found that the area of the lesion tissue around the injury site was larger in aged mice compared with young SCI mice at the same time point. Next, we explored the pathology at 56 days post-SCI. As Figure 1G–H shows, the area of the lesion around the injury site was larger in aged mice compared with young SCI mice at this time point and, conversely, there was less healthy spinal cord tissue in aged mice compared with young mice post-SCI (Figure 1I). Moreover, footprint analysis revealed that stride length and paw rotation were severely impaired in aged mice compared with young mice post-SCI (Figure 1J–L). Taken together, these findings imply that pathological changes in the spinal cord are worse, and neurological functional recovery is more impaired, in aged mice compared with young mice post-SCI.
Figure 2|Aging results in reduced vessel numbers and vascular proliferation after SCI.

Figure 3|Three-dimensional visualization of microvasculature changes in the spinal cords of young and aged mice post-SCI.
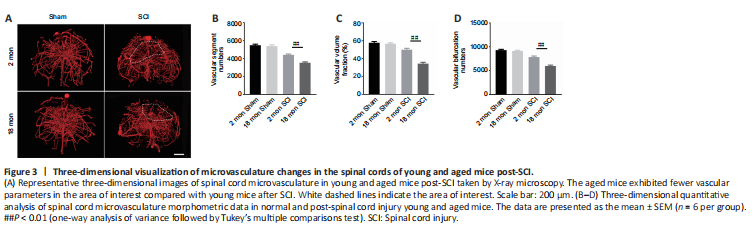
To determine whether the poor recovery in aged mice post-SCI was due to impaired angiogenesis, the blood vessels were visualized by immunostaining. As presented in Figure 2A and B, the aged mice exhibited a lower percentage of newly formed blood vessels (CD31-positive cells) at 56 days post-SCI compared with young mice. Because the greatest degree of vascular proliferation occurs at 7 days post-injury (Cao et al., 2017), this time point was selected to evaluate the proliferation of SCMECs isolated from aged and young mice. As Figure 2C and D shows, immuno-stained spinal cord slices taken 7 days post-SCI exhibited substantially fewer proliferated endothelial cells (Ki-67 and CD31 double-positive cells) in the aged group compared with the young group, suggesting that micro-vessel growth is attenuated in aged mice after SCI. Next, qPCR was conducted to analyze alterations in the mRNA expression levels of four angiogenesis-related genes (ANG-1, BDNF, CTGF, and VEGF) within injured spinal cord tissue from both groups. We found that ANG-1, BDNF, CTGF, and VEGF mRNA levels were markedly higher in the young group compared with the aged group at 7 days post-SCI (Figure 2E–H). Then, comparative X-ray microscope tomography imaging of the spinal cord microvasculature was performed to analyze specific 3D morphological alterations post-SCI in both young and aged mice. As shown in Figure 3A, visualization of the 3D architecture of the spinal cord micro-vessel networks enabled accurate quantification of vascular segment, vascular volume fractions, and vascular bifurcation. Figure 3A depicts 3D images of the spinal cord microvasculature at the T10 levels in control and injured young and aged mice. In the control animals, the quantitative parameters of the spinal cord vasculature were similar in aged mice and young mice. However, at 56 days post-SCI, the vascular parameters of the spinal cord were lower in aged mice than in young mice (Figure 3B–D), which was consistent with our immunostaining results. Collectively, these data suggest that the impaired neurological functional recovery observed in the aged mice may be associated with impaired vascular regeneration.
Figure 4|Metformin promotes SCMEC-mediated angiogenesis in vitro.

Metformin has anti-aging and pro-angiogenic effects in the context of several diseases (Takahashi et al., 2015; Barzilai et al., 2016; Yu et al., 2016; Piskovatska et al., 2020). To determine whether metformin promotes angiogenesis after SCI, we first explored its effects on SCMECs in vitro. First we isolated SCMECs and confirmed their identity by light microscopy and immunofluorescence (Additional Figure 5). Seven days after seeding, SCMEC colonies appeared (Additional Figure 5A), and CD31 immunofluorescence was detected (Additional Figure 5B). Next, we performed scratch and Transwell assays and found that metformin enhanced SCMEC migration horizontally and vertically in a concentration-dependent manner, as shown in Figure 4A–D. Additionally, SCMEC canaliculization increased markedly as the metformin concentration increased, as indicated by the tube formation assay (Figure 4E–G). Of note, 4 mM metformin induced less SCMEC migration and tube formation than 2 mM metformin, suggesting that 2 mM may be the optimal concentration of metformin for promoting SCMEC-mediated angiogenesis.
Figure 5|Metformin promotes neurological functional recovery and tissue repair after SCI in aged mice.
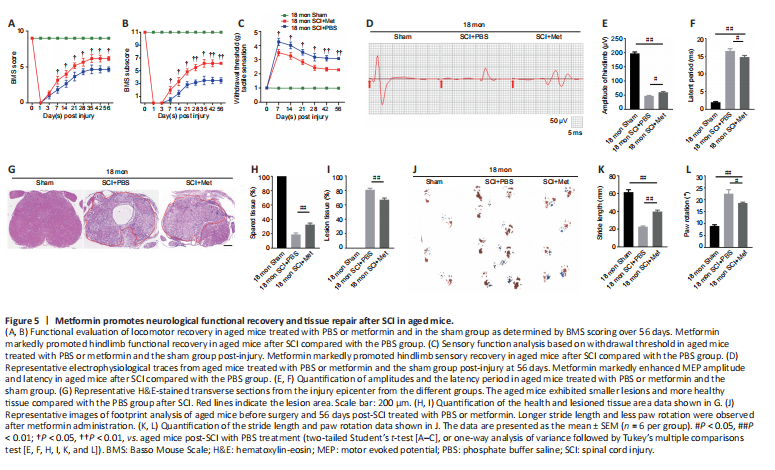
To better illustrate metformin’s role in tissue repair and functional recovery in aged mice after SCI, we systematically evaluated the recovery of hindlimb motor function. First, the BMS score and subscore were determined to assess the recovery of neurological function in the metformin and PBS groups. As shown in Figure 5A and B, the aged mice treated with metformin had markedly higher BMS scores and subscores (starting at 7 days post-SCI) and enhanced motor function than the aged mice treated with PBS post-SCI. Additionally, the aged mice treated with metformin had lower withdrawal thresholds in response to mechanical stimulus than the aged mice treated with PBS post-SCI, suggesting that metformin may promote sensory recovery in aged mice post-SCI (Figure 5C). To better evaluate the role of metformin function in the recovery of motor function, electrophysiological testing was carried out. As shown in Figure 5D, treatment with metformin lead to clearly elevated MEP amplitudes and markedly shorter latency periods relative to treatment with PBS at 56 days post-injury (Figure 5E and F). H&E staining showed that the area of spinal cord tissue with normal integrity at the injury site was markedly increased in the metformin group compared with the PBS group (Figure 5G and H), and the area of injured tissue was dramatically decreased in the metformin group compared with PBS group (Figure 5I). Furthermore, footprint analysis showed that treatment with metformin resulted in a longer stride length and less paw rotation in aged mice at 56-day post-SCI relative to the PBS group (Figure 5J–L). These results suggest that metformin is neuroprotective and significantly improves tissue repair and neurological functional recovery in aged mice post-SCI.
Figure 6|Metformin promotes vascular regeneration in aged mice post-SCI.
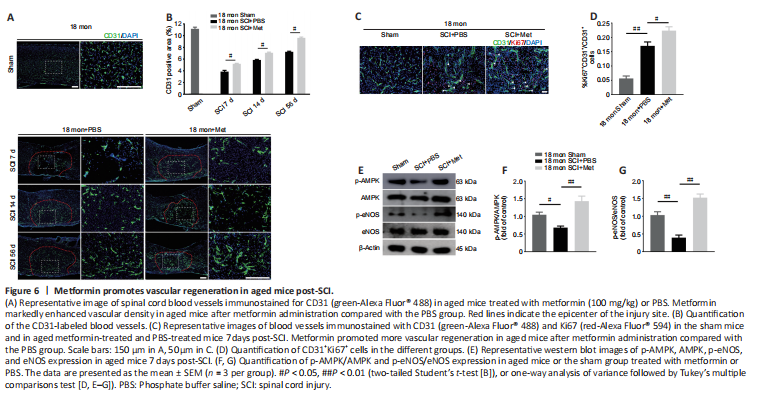
Next, we asked whether the neuroprotective effect of metformin on SCI is due to its promotion of angiogenesis. Immunofluorescence analysis showed that metformin administration markedly increased the percentage of CD31-positive cells at the injury site in aged mice 7, 14, and 56 days after SCI compared with PBS administration (Figure 6A and B). Furthermore, treatment with metformin increased the percentage of CD31 and Ki67 double-positive cells in aged mice at 7 days post-SCI (Figure 6C and D).
To explore the molecular mechanism by which metformin promotes angiogenesis in aged mice post-SCI, we investigated the AMPK/eNOS pathway, which has been shown to have a beneficial effect on endothelial cells after treatment with metformin (Takahashi et al., 2015; Yu et al., 2016). The AMPK/eNOS pathway was markedly activated in the spinal cord of aged animals 7 days post-SCI after treatment with metformin, as confirmed by western blotting analysis (Figure 6E–G). Collectively, these results suggest that activation of the AMPK/eNOS pathway may be involved in the metformin-mediated angiogenesis observed in aged mice post-SCI.
Figure 7|Inhibition of the AMPK/eNOS pathway attenuates the beneficial effects of metformin on angiogenesis in SCMECs in vitro.
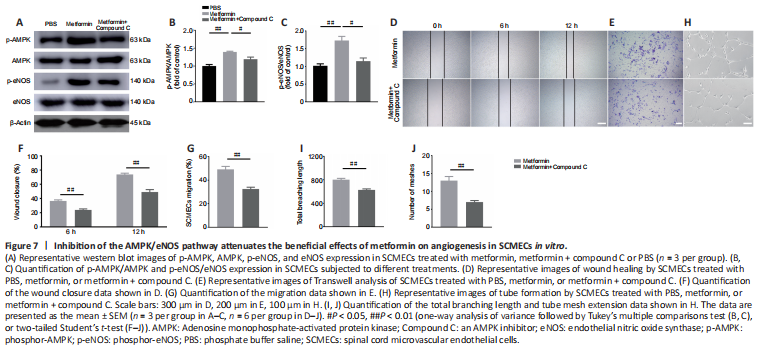
To determine whether the AMPK/eNOS pathway affects SCMEC function after treatment with metformin, AMPK expression was inhibited in vitro using compound C. First, we confirmed by western blotting that activation of the AMPK/eNOS pathway was blocked by treatment with compound C (Figure 7A–C). Then, we performed scratch and Transwell assays and found that compound C abolished the horizontal and vertical SCMEC migration caused by treatment with 2 mM metformin (Figure 7D–G). Compound C also robustly repressed SCMEC canaliculization, as determined by quantification of tube formation (Figure 7H–J). These findings indicate that inhibition of the AMPK/eNOS pathway attenuated the pro-angiogenic effects of metformin on SCMECs in vitro.
Figure 8|Inhibition of the AMPK/eNOS pathway abolishes the beneficial effects of metformin on functional recovery in the aged mice following injury.
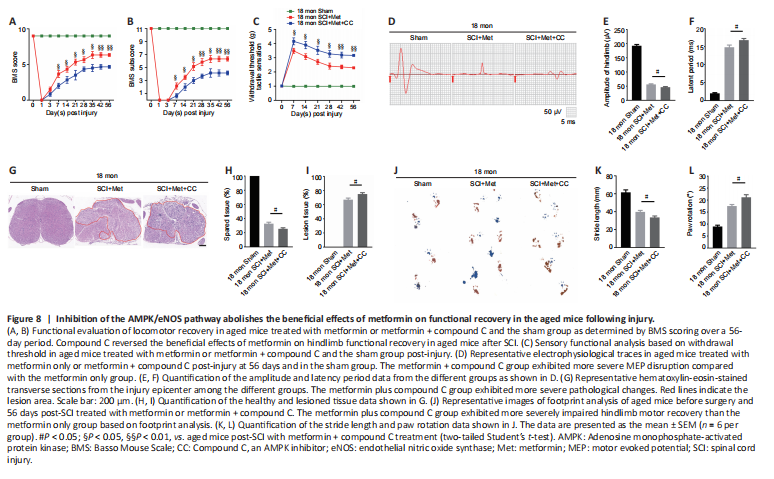
As described above, the AMPK inhibitor compound C attenuates the ability of metformin to promote SCMEC migration and tube formation in vitro. To explore the effect of compound C on neurological functional recovery and angiogenesis, next we intraperitoneally injected compound C and metformin into aged mice after SCI. We first asked whether compound C affects the tissue architecture of the spinal cord before SCI and 56 days post-SCI. As shown in Additional Figure 6, treatment with compound C did not affect the tissue architecture of spinal cord before or after SCI in aged mice. Next, we conducted behavioral assessments (the BMS scoring system and Von-Frey test) over an 8-week observation period to evaluate motor and sensory functional recovery. As shown in Figure 8A and B, BMS scores and subscores were clearly lower in the metformin plus compound C treatment groups than in the metformin only groups beginning at 7 days post-SCI (Figure 8A and B). The withdrawal thresholds in response to mechanical stimuli were also increased in aged mice treated with metformin plus compound C relative to those treated with metformin only, suggesting that compound C abolished the beneficial effect of metformin on motor and sensory functional recovery in aged mice post-SCI (Figure 8C). Electrophysiological analysis showed that, in aged mice post-SCI, the combined treatment group had markedly worsened MEP amplitudes and latency periods compared with metformin only group (Figure 8D–F). In addition, histological analysis revealed less preservation of spinal cord integrity following combined treatment compared with treatment with metformin only (Figure 8G and H). The opposite was true for the lesioned area: the injured area of the spinal cord following treatment with metformin plus compound C was markedly increased compared with metformin only (Figure 8I). Furthermore, footprint analysis demonstrated that the aged mice treated with metformin had a much longer stride length and less paw rotation at 56 days post-SCI, while treatment with metformin plus compound C inhibited hindlimb functional recovery (Figure 8J–L). Thus, compound C inhibits the ability of metformin to promote neurological functional recovery post-SCI in aged mice.
Figure 9|Inhibition of the AMPK/eNOS pathway reverses the beneficial effects of metformin on angiogenesis in aged mice following injury.
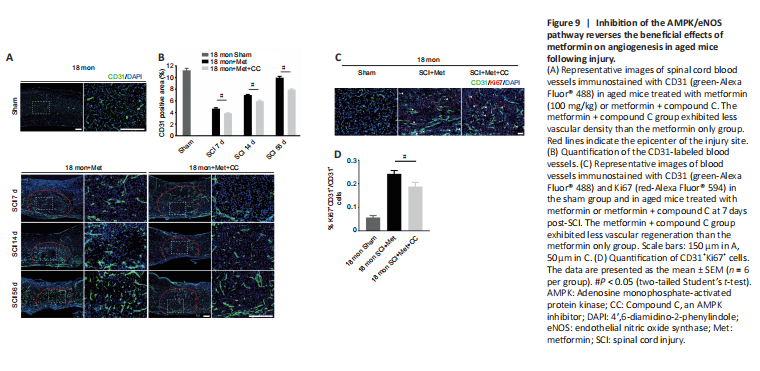
To further explore the role of the AMPK/eNOS pathway in metformin-promoted angiogenesis in vivo, we used immunofluorescence to detect vascular formation and endothelial cell proliferation following treatment with metformin plus compound C. Both the vascular ratio and endothelial cell proliferation at the injury site were reduced after treatment with compound C compared with treatment with metformin alone in aged mice after SCI (Figure 9A–D). These results suggest that compound C inhibits the ability of metformin to promote angiogenesis and neurological functional recovery in aged mice post-SCI.