脑损伤
-
Figure 1|PHACTR-1 expression in the brain after TBI.

To explore the expression changes of PHACTR-1, western blotting was used to determine the overall expression level of PHACTR-1 after TBI. The PHACTR-1 protein level was higher in the TBI group than in the sham group (P < 0.05, P < 0.01), and this higher expression level was maintained for 7 days (Figure 1A and B). Immunofluorescence showed that the cellular localization of PHACTR-1 was altered after TBI: it was expressed only in endothelial cells and neurons in the sham group but was expressed in astrocytes and microglia in the TBI group (Figure 1C). This may explain the increased overall expression of PHACTR-1 after TBI and suggests a potential correlation between increased PHACTR-1 expression and function after TBI.
Figure 2|Effect of AAV targeting PHACTR-1 on the contusion point after TBI.

To overexpress and knockdown the PHACTR-1, we transfected mouse brain tissue with AAV by subcortical three-point injection. After 4 weeks of normal feeding, none of the mice exhibited abnormal vital signs or had died. To determine whether transfection was successful, frozen sections of brain tissue from a randomly selected mouse were observed under a microscope. Many green fluorescent points representing transfected virus were seen at the junction between the cortex and the hippocampus (Figure 2A).
PHACTR-1 expression in the different AAV transfection groups was detected by western blotting 3 days after establishment of the TBI model by CCI. There was no significant difference in PHACTR-1 expression between the TBI, TBI + NC-OE, and TBI + NC-RNAi groups (P > 0.05). However, PHACTR-1 expression was significantly higher in brain tissue from the TBI + PHACTR-1-OE group than that from the TBI group (P < 0.01), and PHACTR-1 expression in the TBI + PHACTR-1-RNAi group was significantly lower than that seen in the TBI group (P < 0.01; Figure 2B and C). These findings suggest that the transfected mice could still achieve the expected expression effect of PHACTR-1 after the establishment of CCI model.
Figure 3|Effect of PHACTR-1 on brain edema and BBB integrity 3 days after TBI.

Figure 4|Effect of PHACTR-1 on AQP-4 and ICAM-1 expression in injured brain tissues 3 days after TBI.
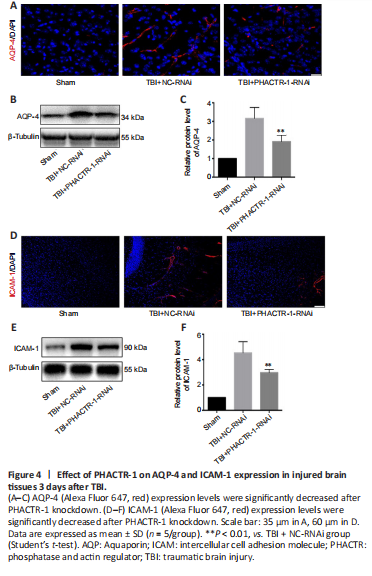
We calculated brain tissue water content using a wet-dry method. Compared with the TBI group, the water content was similar in the TBI + NC-RNAi group (P > 0.05); however, it was decreased in the TBI + PHACTR-1-RNAi group (P < 0.05) (Figure 3A). Thus, the TBI + NC-RNAi group was used in place of the TBI group for subsequent tests. Less EB dye extravasation from the contusion lesion was seen in the TBI + PHACTR-1-RNAi group compared with in the TBI + NC-RNAi group (Figure 3B), as confirmed by spectrophotometry (P < 0.01; Figure 3C). Tight junctions (TJs) act as paracellular barriers to diffusion, and TJ disruption leads to an increase in BBB permeability and vasogenic edema. ZO-1 and occludin are important components of TJs and play an important role in maintaining BBB integrity (Jing et al., 2020). We used immunofluorescence and western blotting to show that ZO-1 (P < 0.05) and occludin (P < 0.01) expression levels were significantly increased in the TBI + PHACTR-1-RNAi group compared with the TBI + NC-RNAi group (Figure 3D–F). AQPs are a group of proteins related to transmembrane transport of fluid, and AQP-4 is the most abundant AQP isoform (Yuan et al., 2016). ICAM-1 binds to some antigens in leukocytes to regulate the infiltration of leukocytes into the brain parenchyma (Michinaga and Koyama, 2019). Immunofluorescence and western blotting revealed that the increased AQP-4 and ICAM-1 immunopositivity after TBI was significantly reduced by PHACTR-1 knockdown (both P < 0.01; Figure 4). Together, these results show that inhibition of PHACTR-1 expression promotes BBB integrity and reduces brain water content, thereby maintaining normal physiological function in the brain.
Figure 5|Effect of PHACTR-1 on neuroinflammation in injured brain tissues 3 days after TBI.
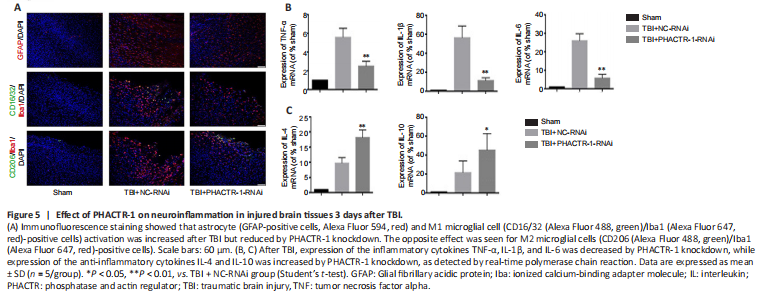
We examined astrocyte and microglial activation and the mRNA levels of inflammatory cytokines to assess the degree of neuroinflammation after TBI. Immunofluorescence staining for GFAP and CD16/32/Iba1 indicated significantly reduced astrocyte and microglial activation in the TBI + PHACTR-1-RNAi group compared with the TBI + NC-RNAi group, while CD206/Iba1 expression was significantly increased (Figure 5A). In addition, the mRNA levels of the inflammatory cytokines TNF-α, IL-1β, and IL-6, were decreased (all P < 0.01; Figure 5B), whereas those of the anti-inflammatory cytokines IL-4 (P < 0.01) and IL-10 (P < 0.05) were increased (Figure 5C). These findings clearly show that the neuroinflammation induced by TBI decreased when PHACTR-1 expression was inhibited.
Figure 6|Effect of PHACTR-1 on neuronal apoptosis and brain volume loss 7 days after TBI.
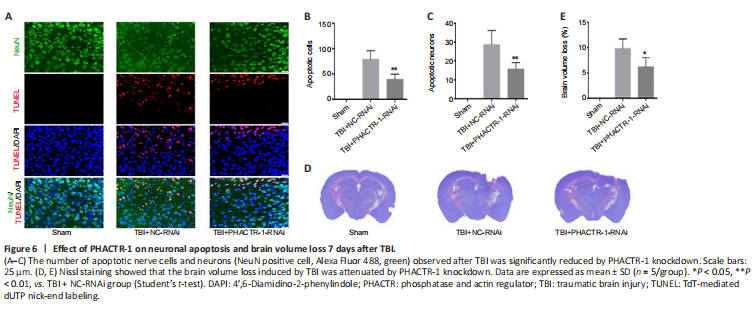
We used TUNEL/NeuN staining to detect nerve cell apoptosis in the three groups. After staining, we found no apoptotic cells in the sham group. However, in the TBI + NC-RNAi group, large numbers of apoptotic neurons and other nerve cells were observed. After PHACTR-1 knockdown, the number of apoptotic neurons and other nerve cells was significantly reduced (both P < 0.01; Figure 6A–C). Nissl staining was used to determine the loss of volume in injured brain tissues after TBI. The percentage of volume loss was about 10% in the TBI + NC-RNAi group, compared with about 6% in the TBI + PHACTR-1-RNAi group (P < 0.05), indicating that PHACTR-1 knockdown reduced brain volume loss after TBI (Figure 6D and E).
Figure 7|Effect of PHACTR-1 on NF-κB/p65 expression in injured brain tissue 3 days after TBI.
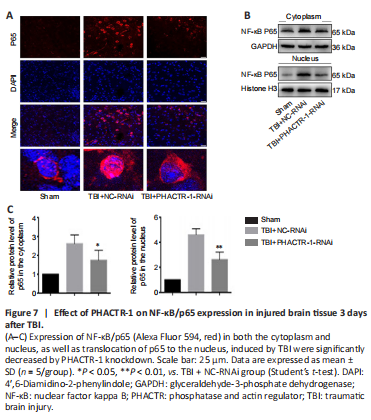
The NF-κB signaling pathway is activated after TBI, and p65, the core protein of the NF-κB complex, is translocated from the cytoplasm into the nucleus, promoting the initiation of apoptosis (Teng et al., 2017; Mettang et al., 2018; Kong et al., 2019). Immunofluorescence staining and western blotting were used to assess p65 expression levels. p65 protein expression levels were low in the sham group, and both p65 expression and translocation to the nucleus were significantly increased in the TBI + NC-RNAi group. PHACTR-1 knockdown inhibited p65 expression and translocation to the nucleus (Figure 7A). Western blotting analysis of p65 localization to the cytoplasm and nucleus showed that PHACTR-1 knockdown reduced activation of the NF-κB signaling pathway after TBI and reduced p65 expression in both the cytoplasm and the nucleus (P < 0.05 and P < 0.01, vs. TBI + NC-RNAi group, respectively, Figure 7B and C).