脑损伤
-
Figure 1|Microglia/macrophages and astrocytes become activated and engulf synapses after TBI.

To evaluate whether activated microglia and astrocytes become phagocytic after TBI, brain tissue samples were co-stained with Mac-2, a specific marker for phagocytosis (Shi et al., 2021), Iba-1, a microglia/macrophage marker (Li et al., 2022b), and GFAP, an astrocyte marker (Zhang et al., 2022), at 1, 3, 7, and 14 days after TBI (Figure 1A). We detected Mac-2+/Iba-1+ and Mac-2+/GFAP+ cells in the peri-contusive areas, suggesting that microglia/macrophages and astrocytes were activated after TBI (Figure 1B). The numbers of Mac-2+/Iba-1+ and Mac-2+/GFAP+ cells remained elevated up to 14 days after TBI, although their levels varied at different time points. More than 32% of microglia/macrophages remained phagocytic from day 1 to day 14 after TBI (Figure 1C); however, less than 25% of astrocytes were phagocytic from day 1 to day 7 after TBI, whereas 35% of astrocytes become phagocytic 14 days after TBI (Figure 1C).
We next examined whether microglia/macrophage- and astrocyte-mediated phagocytosis cause synapse loss in TBI mouse brains. The results showed that microglia/macrophages and astrocytes engulfed both presynaptic elements labeled with SYP and postsynaptic elements labeled with Homer-1 (Shi et al., 2021) 14 days after TBI (Figure 1D). Confocal imaging showed SYP+ and Homer-1+ signals within Iba-1+ and GFAP+ cells, suggesting that both pre- and postsynaptic elements were phagocytosed by microglia/macrophages and astrocytes. We then compared the number of synaptic puncta in microglia/macrophages and astrocytes and found that both cell types engulfed synapses to a similar extent 14 days after TBI (Figure 1E).
TEM imaging confirmed that activated microglia/macrophages and astrocytes both engulfed synaptic elements in the peri-contusive areas of TBI mice (Figure 1F). Statistical analysis showed that both cell types phagocytosed synaptic materials to a similar extent. We also noted similar levels of multivesicularity and irregular membrane morphology among microglia/macrophages and astrocytes, indicating vesicle degradation (Figure 1G).
Figure 2|MERTK expression is upregulated, mainly in microglia/macrophages and astrocytes, in the mouse brain 14 days after traumatic brain injury.
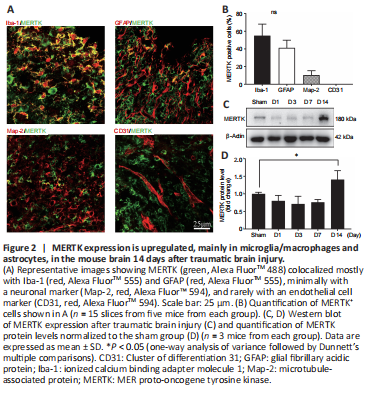
To determine whether MERTK is involved in microglia/macrophage- and astrocyte-mediated engulfment of synapses after TBI, we assessed MERTK expression at various time points after TBI via immunostaining and western blot. The results showed that MERTK was expressed mostly in Iba-1+ and GFAP+ cells, and at low levels in MAP2+ and CD31+ cells, in the mouse brain 14 days after TBI (Figure 2A and B). In addition, MERTK expression was higher 14 days after injury in the TBI group compared to the sham group (Figure 2C and D).
Figure 4|Astrocyte-specific or microglia/macrophage-specific MERTK knockout reduces synapse engulfment and increases synaptic protein expression after traumatic brain injury.

The subacute stage of TBI is the period from 3 days to 2 months after injury (Chitturi et al., 2019; Izzy et al., 2019). We found that MERTKKO in microglia/macrophages or astrocytes decreased phagocytosis of SYP+ and Homer-1+ synaptic elements compared to the controls (Figure 4A–D). We further investigated SYP and Homer-1 levels in synapses in the peri-contusive areas by western blotting (Figure 4E) and found that the levels of both proteins were higher in the knockout groups compared to the controls 14 days after TBI, which is consistent with our immunostaining data (Figure 4F). MERTK knockout in microglia/macrophages and astrocytes showed a similar reduction in synapse engulfment in mice after TBI.
Immune-TEM data also confirmed that microglia/macrophages and astrocytes mediated phagocytosis of synaptic elements. Both astrocytes (identified by GFAP immunolabeling) and microglia/macrophages (identified by Iba-1 immunolabeling) exhibited phagocytic inclusions within their cytoplasm 14 days of TBI (Figure 4G). In addition, phagocytic inclusions were reduced in microglia/macrophages- or astrocyte-specific MERTKKO mice compared to controls, indicating a reduction in phagocytosis (Figure 4H and I).
Figure 6|Synaptic materials are digested by lysosomes in microglia/macrophages and astrocytes after traumatic brain injury.
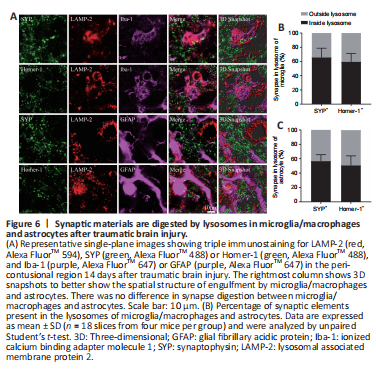
To confirm that microglia/macrophages and astrocytes digest synapses through lysosomal-mediated degradation, as we concluded based on the irregular membrane morphology observed by TEM, we performed triple immunostaining of microglia/macrophages or astrocytes with LAMP2, a lysosome marker, and Homer-1 or SYP. The results showed that SYP+ and Homer-1+ signals colocalized with Lamp-2+ signals in microglia/macrophages and astrocytes, suggesting that synaptic materials were being degraded by lysosomes (Figure 6A). However, some SYP+ and Homer-1+ signals did not colocalize with Lamp-2+ signals (Figure 6B), indicating synaptic elements were being transferred to lysosomes.
Figure 7|Both inhibitory and excitatory synapses are engulfed by microglia/macrophages and astrocytes after TBI.
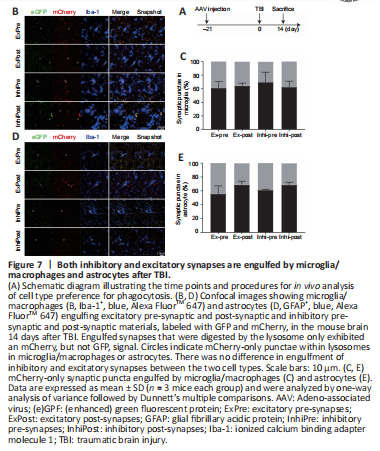
To further determine which types of synaptic material were phagocytosed in TBI, we used recently developed synapse phagocytosis sensors (Lee et al., 2021). AAV carrying fluorescent phagocytosis reporters for excitatory pre-synapses (hsyn-Synaptophysin-mCherry-eGFP), inhibitory pre-synapses (GAD67-Synaptophysin-mCherry-eGFP), excitatory post-synapses (hsyn-PSD95-mCherry-eGFP), and inhibitory post-synapses (hsyn-Gephyrin-mCherry-eGFP) were injected into the cortical region of the mouse brain. When synaptic elements were digested in the lysosomes, GFP fluorescence was attenuated, while mCherry fluorescence was more stable (Figure 7A and B). All four kinds of synaptic elements were found in microglia/macrophages and astrocytes (Figure 7B and D), and the percent of each type was similar between microglia/macrophages and astrocytes (Figure 7C and E), indicating that neither cell type preferentially engulfs excitatory or inhibitory synapses.