脊髓损伤
-
Figure 2| Expression analysis of MIF and CCL2 at lesion sites following SCI.
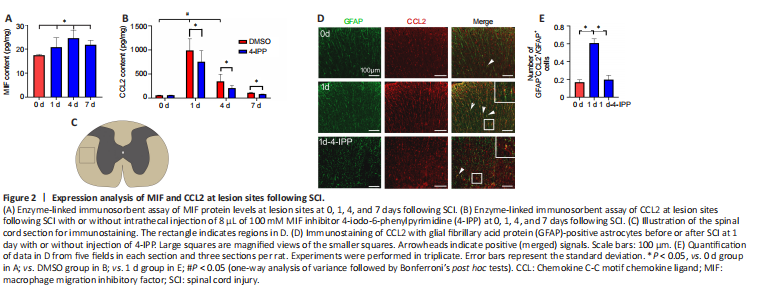
CCL2 plays crucial roles in changes of the lesion microenvironment and affects neuronal and non-neuronal pathophysiological functions (Knerlich-Lukoschus and Held-Feindt, 2015). To understand whether MIF contributes to the production of CCL2, which impacts the neuropathology, we next examined the relationship between the expression of CCL2 and proinflammatory MIF following SCI. ELISA results showed that MIF levels significantly increased at the lesion site at 1, 4, and 7 days after SCI (1 day vs. 0 day: P = 0.0284; 4 days vs. 0 day: P = 0.0217; 7 days vs. 0 day: P = 0.0159; Figure 2A). Consistent with the results above, the production of CCL2 was also remarkably elevated, with a peak at 1 day (1 day vs. 0 day: P < 0.0001; 4 days vs. 0 day: P = 0.0004; Figure 2B). Notably, administration of the MIF inhibitor 4-IPP at the lesion site resulted in a remarkable reduction of CCL2 protein levels at various days following cord contusion (1 day vehicle vs. 1 day 4-IPP: P = 0.0219; 4 days vehicle vs. 4 days 4-IPP: P = 0.0476; 7 days vehicle vs. 7 days 4-IPP: P = 0.0183; Figure 2B). Immunostaining revealed that CCL2 primarily colocalized with GFAP-positive astrocytes before and after SCI (Figure 2C–E), though with less distribution in the neuron, microglia, and endothelial cells (Additional Figure 1). The expression of CCL2 in astrocytes was significantly attenuated by the MIF inhibitor (0 day vs. 1 day: P < 0.0001; 1 day vehicle vs. 1 day 4-IPP: P < 0.0001; Figure 2D and E). These data indicate that SCI-induced expression of MIF is involved in facilitating CCL2 production from astrocytes.
Figure 4| MIF promotes CCL2 production from astrocytes.
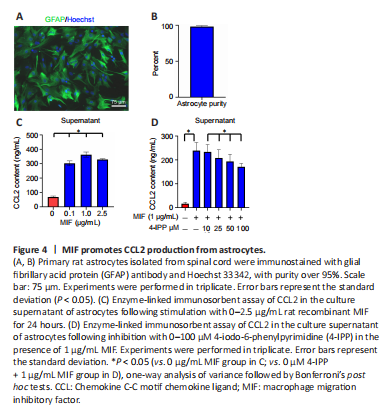
To more closely examine the effects of MIF on astrocytic production of CCL2, primary astrocytes with purity over 95% were stimulated with 0–2.5 μg/mL MIF recombinant protein for 24 hours (Figure 4A and B). ELISA showed that treatment of cells with various concentrations of MIF robustly promoted the production of CCL2 from astrocytes (Figure 4C). Addition of the MIF inhibitor 4-IPP in the presence of 1 μg/mL MIF recombinant protein for 24 hours significantly decreased the production of CCL2 in a concentration-dependent manner (25 μM 4-IPP vs. 0 μM 4-IPP: P = 0.0033; 50 μM 4-IPP vs. 0 μM 4-IPP: P = 0.0058; 100 μM 4-IPP vs. 0 μM 4-IPP: P = 0.03; Figure 4D). These data indicate that MIF promotes the production of CCL2 from astrocytes.
Figure 6| CCL2 facilitates the migration of BV2 cells.

BV2 cells are mouse microglia cells and microglia are the resident immune cells in the spinal cord. We used BV2 cells to investigate the effects of CCL2 on the microglia. To examine the effects of MIF-mediated CCL2 production from astrocytes on the recruitment of inflammatory cells, we performed migration assays with BV2 cells co-cultured with astrocytes with or without knockdown of CCL2. The most efficient siRNA (siRNA2) among three siRNAs targeting CCL2 was selected (Figure 6A). Chemokines show different chemotaxis effects on leukocytes in different activation states (Zhou et al., 2018). Thus, BV2 cells were stimulated with either 0.5 μg/mL LPS or 20 ng/mL recombinant rat IL-4 protein for 24 hours prior to the assay; LPS resulted in a transition of the cells to M1 phenotype and IL-4 caused induction of the M2 phenotype, as identified by iNOS and Arginase1 expression (Murray et al., 2014; Figure 6B and C). BV2 cells co-cultured with astrocytes for 24 hours in the presence of 1 μg/mL MIF stimulation exhibited a remarkable migratory ability in comparison with the control. However, knockdown of CCL2 expression in astrocytes for 24 hours before co-culture significantly attenuated the increased cell migration (siRNA2 vs. Scramble: P < 0.05; Figure 6D–F). BV2 cells pretreated with IL-4 were more susceptible to astrocyte-mediated migration, whereas LPS resulted in a remarkable inhibitory effect (Figure 6D–F). These data indicate that MIF-mediated CCL2 expression in astrocytes is involved in promoting the migration of microglia.
Figure 7| MIF inhibitor 4-IPP restrains the accumulation of microglia/macrophages at lesion site of the cord following SCI.

To further explore the role of MIF-induced CCL2 production from astrocytes in the neuropathology following SCI, we intrathecally injected the MIF inhibitor 4-IPP into the lesion site immediately after spinal cord injury. Immunostaining with Iba-1 antibody demonstrated that the accumulation of microglia at lesion site at 1 and 4 days after SCI was significantly reduced by the 4-IPP inhibitor (1 day vehicle vs. 1 day 4-IPP: P = 0.0007; 4 day vehicle vs. 4 day 4-IPP: P = 0.00014; Figure 7A and B), concomitant with the decrease of CCL2 protein levels (Figure 2B). Western blot of CD68, the specific marker of macrophages, showed that 4-IPP significantly decreased infiltration of macrophages at the lesion site (4 days vehicle vs. 4 days 4-IPP: P = 0.0009; 7 days vehicle vs. 7 days 4-IPP: P < 0.0001; Figure 7C).
Figure 8| MIF inhibitor 4-IPP promotes the locomotor function recovery of rats following SCI.
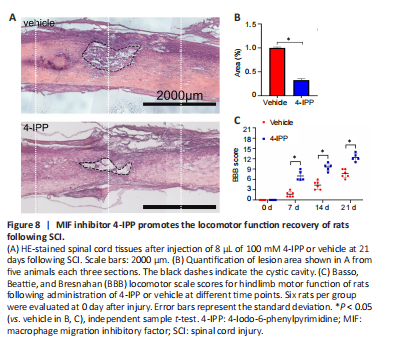
Excessive inflammation is associated with formation of the cord cystic cavity. To explore the biological importance of 4-IPP in the secondary tissue damage following SCI, we performed HE staining of the lesion area after cord contusion at 21 days. The results demonstrated that 4-IPP reduced the size of the cystic cavity in comparison with the vehicle (Figure 8A and B).
We next evaluated the functional recovery after inhibition of MIF after SCI in rats by BBB scores. The results showed that 4-IPP significantly improved the hindlimb locomotor function of rats from 7 days onwards compared with the vehicle (7 day vehicle vs. 7 days 4-IPP, 14 days vehicle vs. 14 days 4-IPP, 21 days vehicle vs. 21 days 4-IPP: P < 0.0001; Figure 8C). These data indicate that inhibition of MIF following SCI decreases CCL2 production and recruitment of microglia/macrophages at lesion site, thereby improving the functional recovery of rats after SCI.