脊髓损伤
-
Figure 4| Inhibition of tau contributes to M2 polarization in the acute phase after SCI.
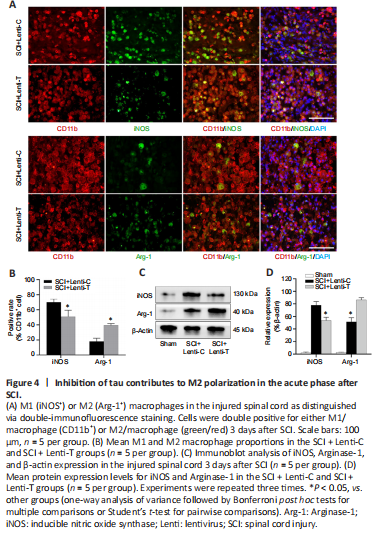
To determine the effect of tau inhibition on the polarization of macrophages in SCI, macrophage phenotypes was assessed on day 3 after SCI. CD11b is a typical microglia (macrophage) marker, and iNOS and Arginase-1 are used to distinguish M1 and M2 sub-populations, respectively. Immunofluorescence staining of spinal cord sections showed that, compared with the SCI + Lenti-C group, the SCI + Lenti-T group had a significantly lower percentage of iNOS-positive M1 macrophages and a higher proportion of Arginase-1-expressing M2 macrophages among the CD11b-positive cells (P < 0.05; Figure 4A and B and Additional Figure 1). The expression levels for iNOS and Arginase-1 at the lesion site were assessed by immunoblot analysis on day 3 after injury. Compared with that in the SCI + Lenti-C group, iNOS expression was significantly lower in the SCI + Lenti-T group, but Arginase-1 expression was significantly higher (P < 0.05; Figure 4C and D). To summarize, these results indicate that tau inhibition likely helped promote M2 macrophage polarization in the injured spinal cord.
Figure 5|Inhibition of tau alleviates oxidative stress in the acute phase after SCI.
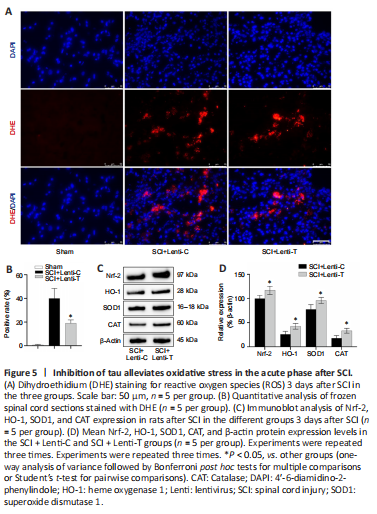
Oxidative stress is a common and critical pathologic process that occurs after SCI in which increased ROS derived from M1 macrophages causes additional damage to spinal cord tissue (David and Kroner, 2011; Liu et al., 2020; Zhang et al., 2021). Because tau inhibition reduced the polarization of M1 macrophages, we speculated that inhibition of abnormal tau played an important role in regulating redox reactions. On day 3 after injury, DHE staining showed that tau inhibition reduced the number of DHE-positive cells after SCI (Figure 5A and B). Subsequently, immunoblot analysis revealed that tau inhibition also increased the expression of critical enzymes (Nrf2, HO-1, CAT, and SOD1) that help protect against oxidative stress (Figure 5C and D). Collectively, knock-down of tau was beneficial for alleviating oxidative stress after SCI.
Figure 6|Inhibition of tau enhances the survival of residual cells around the lesion site after SCI.
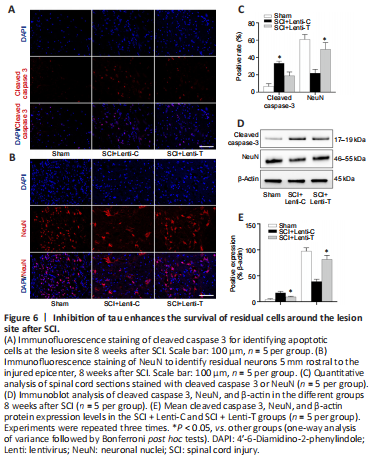
Eight weeks after SCI, apoptosis levels and the survival of residual cells in the injured spinal cord were evaluated by immunofluorescence staining and immunoblot analysis (Chen et al., 2016). Pronounced cell apoptosis with positive-cleaved caspase-3 was observed in the tissue sections containing the injury epicenter, but significantly less cell apoptosis was observed in the SCI + Lenti-T group than in the SCI + Lenti-C group (P < 0.05; Figure 6A and C). Immunoblot analysis revealed similar expression levels of cleaved caspase-3 (Figure 6D and E). NeuN, a specific nucleoprotein found in neurons, was used to assess the residual number of neurons around the epicenter. The number of NeuN-positive neurons located 5 mm rostral and caudal from the lesion epicenter were examined via immunofluorescent staining and immunoblot analysis to determine whether tau inhibition protects the residual neurons. We found significantly more cells with positive-NeuN staining (Figure 6B and C) in the SCI + Lenti-T group than in the SCI + Lenti-C group (P < 0.05). A similar NeuN-protein expression pattern was detected by immunoblot analysis (Figure 6D and E). In summary, these results indicate that tau inhibition suppressed cell apoptosis and enhanced the survival of cells around the lesion site.
Figure 7|Inhibition of tau improves neural regeneration and reduces astrocyte infiltration after SCI.
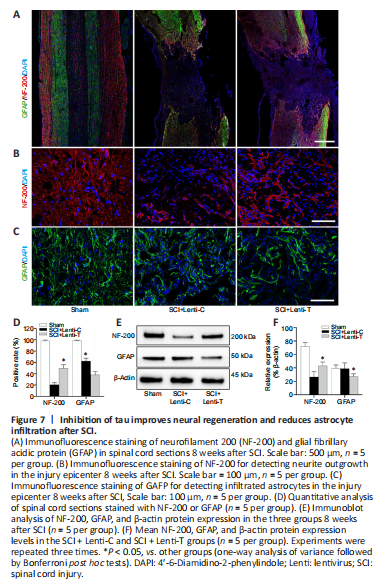
The proportion of cells expressing NF-200 was higher in the SCI + Lenti-T group than in the SCI + Lenti-C group (P < 0.05), while the number of GFAP-positive cells were much higher in the SCI + Lenti-C group than in the SCI + Lenti-T group (P < 0.05; Figure 7A–D). The Western blot results for NF-200 and GFAP indicated a similar pattern (Figure 7E and F). Thus, knock-down of tau increased NF-200 expression and reduced GFAP expression. These results suggest that inhibition of tau might promote neurogenesis and decrease astrocyte formation.
Figure 8| Inhibition of tau facilitates axonal regeneration after SCI.
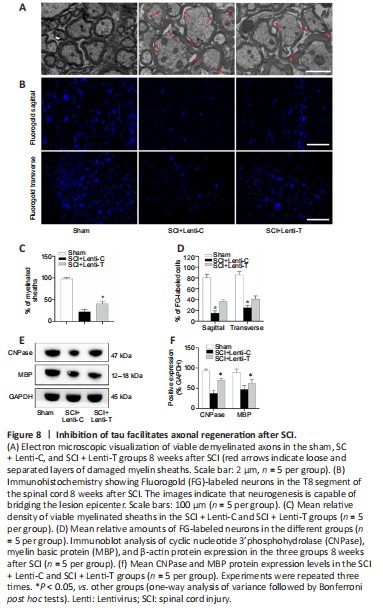
In addition to neuronal regeneration, axonal regeneration is another crucial element for effective SCI treatment. To assess axon regeneration, EM was used to evacuate axon myelination and immunoblot analysis was used to evaluate the expression of oligodendrocytes markers (cyclic nucleotide 3’phosphohydrolase [CNPase] and myelin basic protein [MBP]). The loss of myelin was apparent in both SCI groups. However, the percentage of myelinated axons in the SCI + Lenti-T group was significantly greater than that in the SCI + Lenti-C group (P < 0.05; Figure 8A and C). Immunoblot analysis indicated that the SCI rats treated with Lenti-T expressed significantly more CNPase and MBP than did the Lenti-C treated rats (P < 0.05; Figure 8E and F). Furthermore, we assessed neural circuit plasticity of remyelinated axons via retrograde tracing. In the Sham group, almost all neurons in the ventricolumna were labeled by FG at the level of T8. A significantly smaller number of T8-level neurons were FG-positive in the SCI groups (P < 0.05). However, the number of FG-positive neurons in the SCI + Lenti-C group was significantly higher than that in the SCI + Lenti-C group, (P < 0.05; Figure 8B and D). Therefore, we can conclude that tau inhibition after SCI facilitated axonal regeneration.
Figure 9| Inhibition of tau enhances neurological recovery by 8 weeks after SCI.
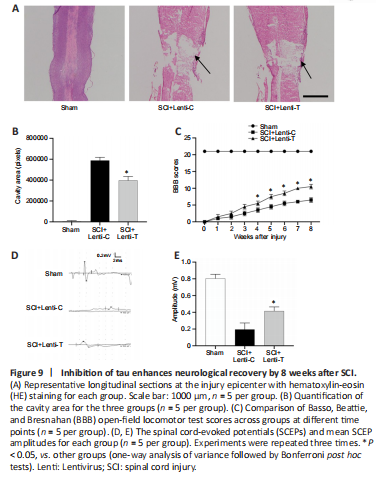
First, the SCI cavity was assessed by HE staining 8 weeks postoperatively to determine the extent of tissue repair. We found that the cavity area was significantly smaller in the SCI + Lenti-T group than in the SCI + Lenti-C group (P < 0.05; Figure 9A and B). Furthermore, locomotor recovery was evaluated via BBB scores. Rats in the Sham group maintained 21 points at all time points. All rats subjected to SCI scored zero on the first day after injury, which indicated successful SCI. All injured rats achieved higher BBB scores from the 1st week to the 8th week, and the rats treated with Lenti-T achieved notably better BBB scores than those treated with Lenti-C at each evaluation after the 4th week (all P < 0.05; Figure 9C). Subsequent, electrophysiological analysis indicated that locomotor recovery correlated with SCEP responses. While SCEPs in both SCI groups were weak 8 weeks after SCI compared with those of the Sham group (P < 0.05), they were significantly stronger in the SCI + Lenti-T group than in the SCI + Lenti-C group (P < 0.05; Figure 9D and E). These results indicated that tau inhibition facilitated the recovery of locomotor function in SCI rats.