脑损伤
-
Figure 2|Neuroprotective and anti-inflammatory effects of meloxicam after 48 hours of reperfusion.
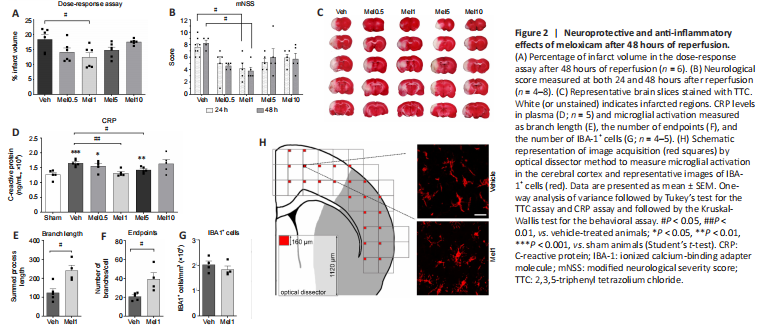
To set up the optimal neuroprotective dose of meloxicam, a dose-response assay measuring the infarct volume after 48 hours of reperfusion was performed (Figure 2A). The different dosages displayed a U-shaped curve where the neuroprotective effects reached statistical significance at 1 mg/kg of meloxicam (Mel1) (Veh vs. Mel1, P = 0.0132). Consistently, behavioral data at both 24 and 48 hours after reperfusion only displayed significant improvement for 1 mg/kg of meloxicam (Veh 24 h vs. Mel1 24 h, P = 0.0371; Veh 48 h vs. Mel1 48 h, P = 0.040) (Figure 2B). Representative brain slices stained with TTC are observed in Figure 2C.
After 48 hours of reperfusion, the levels of CRP were used to measure systemic inflammation in plasma. The range of doses assayed showed a U-shaped curve and only animals treated with 1 and 5 mg/kg of meloxicam reached significantly lower CRP levels than vehicle animals (Veh vs. Mel1, P = 0.0039; Veh vs. Mel5, P = 0.0375) (Figure 2D). To corroborate the anti-inflammatory effect, the microglia activation in the ischemic hemisphere (ipsilateral) was only measured after the administration of the neuroprotective dose (1 mg/kg of meloxicam). The total branch length and endpoints of IBA-1+ cells significantly increased in treated animals (Veh vs. Mel1, P = 0.010 and P = 0.02 respectively) (Figures 2E and F, respectively). No changes in the number of IBA-1+ cells were detected (Veh vs. Mel1, P = 0.385) (Figure 2G).
Figure 3|Long-term neuroprotection of meloxicam treatment.
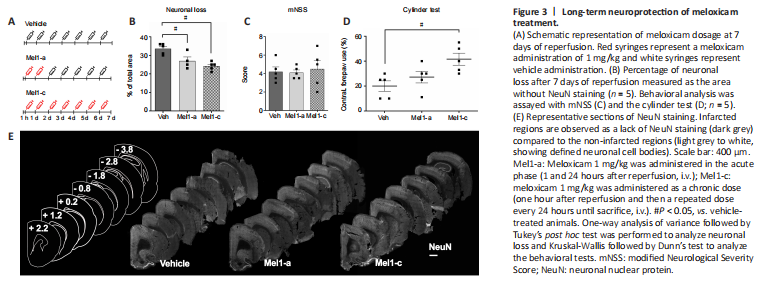
To determine if neuroprotection exerted by 1 mg/kg is maintained after 7 days of reperfusion, we measured the extent of the stroke using the absence of neurons as a proxy of damaged regions after acute (Mel1-a) and chronic (Mel1-c) treatments with meloxicam (Figure 3A and E). Neuronal loss observed in vehicle animals was decreased by the acute and chronic treatments with meloxicam (Veh vs. Mel1-a, P = 0.0247; Veh vs. Mel1-c, P = 0.0017; Figure 3B). Acute and chronic treatments presented similar results. The behavior analysis using the mNSS revealed no differences between vehicle and treated animals (Figure 3C). However, animals chronically treated with meloxicam displayed improved motor behavior in the cylinder test compared to vehicle animals (Veh vs. Mel1-c, P = 0.0138), but not acutely treated animals (Veh vs. Mel1-a, P = 0.2754; Figure 3D).
Figure 4|Astrocyte reactivity in the glial scar in the cerebral cortex.
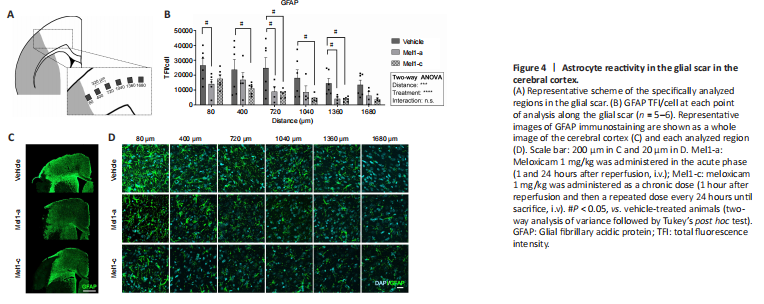
Astrocytes at the edge of the cortical glial scar showed a hypertrophic morphology characterized by engrossed cell bodies with wide branches, which were observed from 80 to 400 μm from the border of the injury and were progressively replaced by less hypertrophied cells further from the edge (Figure 4A, C, and D). The reactivity of astrocytes in the glial scar was quantified as the levels of GFAP immunoreactivity per cell (TFI/cell). This analysis revealed a distance-dependent decrease in the astrocyte reactivity
(F(5, 83) = 5.36, P = 0.0003), attenuated by meloxicam treatment (F(2, 83) = 18.02; P < 0.0001; Figure 4B).
Figure 5|Axonal sprouting after 7 days of reperfusion.
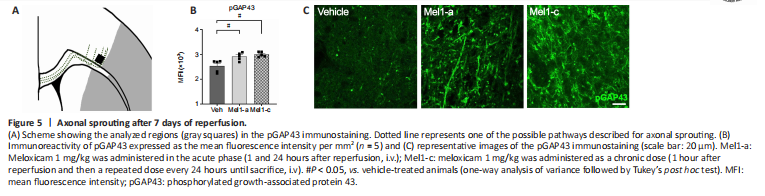
Axonal sprouting was measured in the edge of the glial scar as a molecular parameter of the tissue’s new connectivity attempt (Figure 5A) using pGAP43, a specific marker for axonal sprouting (Kawasaki et al., 2018). The analysis revealed that both chronic and acute treatment with meloxicam significantly increased the levels of pGAP43 after 7 days of reperfusion (Veh vs. Mel1-a, P = 0.0233; Veh vs. Mel1-c, P = 0.0272; Figure 5B and C).
Figure 6|Neuronal progenitors after 7 days of reperfusion.
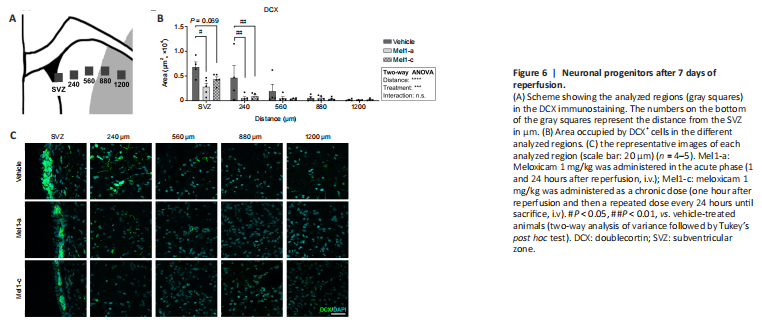
To analyze the effect of meloxicam in neurogenesis, we labeled the neuronal progenitor cells (NPCs) with DCX and analyzed them in both the SVZ (NPCs) and in the striatum (migrating NPCs) (Figure 6A). In general terms, the treatment with meloxicam significantly reduced the area occupied by DCX (two-way ANOVA treatment effect (Meloxicam effect, P = 0.004, F(2,59) = 9.023). The area occupied by DCX cells in the SVZ (expressed as DCX+ area/mm3) was significantly lower in animals treated with an acute dose of meloxicam compared to vehicle animals (SVZ Veh vs. SVZ Mel1-a, P = 0.026). Chronic treatment with meloxicam also decreased the area of DCX cells in the SVZ, but we failed in finding significance (P = 0.069). The closest region to the SVZ analyzed also displayed significant reductions in meloxicam-treated animals (Veh vs. Mel1-a, P = 0.047; Veh vs. Mel1-c, P = 0.0261; Figure 6B). No differences were observed in the different conditions at 560, 880, and 1200 μm of distance from the SVZ (Figure 6C).