脑损伤
-
Figure 1|Identification of NSCs and characteristics of exosomes from NSCs.
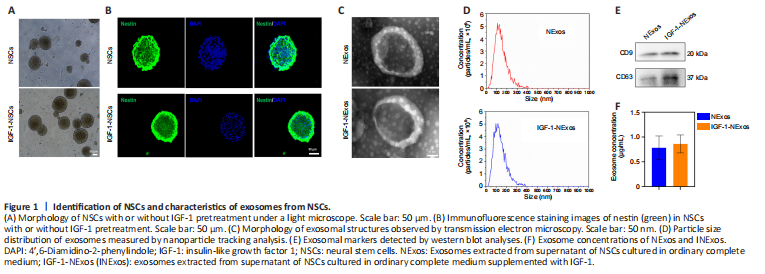
NSCs were isolated from E14 rat brains. There were no differences in the morphology of NSCs with or without IGF-1 pretreatment under light microscopy (Figure 1A), and the NSC marker nestin was expressed in cultured NSCs with/without IGF-1 pretreatment in IF experiments (Figure 1B).
To confirm the effect of INExos on TBI, exosomes in supernatant from NSCs with or without IGF-1 pre-stimulation were obtained by ultracentrifugation and were confirmed by TEM, NTA, and WB analyses. TEM showed homogeneous, spherical, and membrane vesicles of NExos and INExos (Figure 1C). NTA indicated a size distribution of 50–200 nm, with average diameters of 144.3 nm for NExos and 138.9 nm for INExos (Figure 1D). Exosomal surface markers, including CD9 and CD63, were expressed in NExos and INExos using WB (Figure 1E). Furthermore, there was no difference in exosome concentrations in samples derived from NSCs with or without IGF-1 (P > 0.05). These results suggested that NExos and INExos were similar in terms of morphology, particle size, and proteins, indicating that IGF-1 pretreatment had no effect on the exosomal profile of NSCs.
Figure 2|Characteristics of 3D-CC-INExos scaffolds.
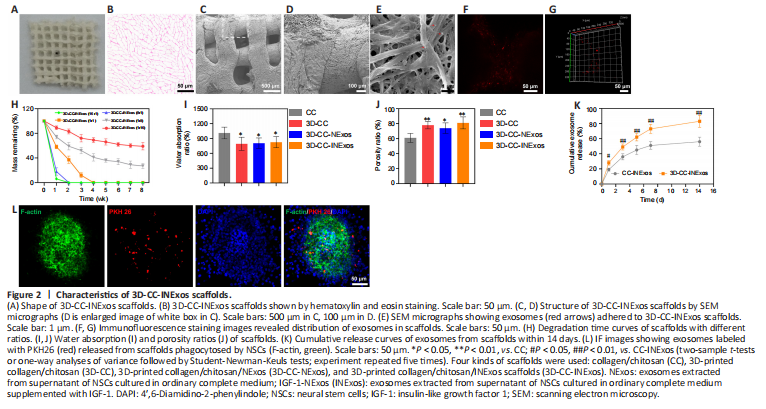
The morphological characteristics of the 3D-printed scaffolds were observed by HE staining, scanning electron microscopy, and CLSM (Figure 2A–F). The morphology was homogeneous according to HE staining (Figure 2B). Scanning electron microscopy showed that the surfaces of the 3D-printed scaffolds were rough and porous (Figure 2C and D), providing an advantage for cell adhesion. Exosomes adhered efficiently to the scaffolds (Figure 2E–G). The scaffold materials are required to have an appropriate degradation rate. We therefore assessed the degradation rates of scaffolds with five different collagen/chitosan mass ratios (16:1, 8:1, 1:1, 1:8, and 1:16) in vivo (Figure 2H). The 16:1 and 8:1 scaffolds showed fast degradation (2 weeks), indicating that they would be unable support the damaged area during the repair process, while the 3D scaffolds with mass ratios of 1:8 and 1:16 had not degraded after 8 weeks, which would result in long-term foreign body residue, which is also not conducive to damage repair. However, 3D-printed scaffolds with a mass ratio of 1:1 were degraded after 4 weeks, which indicated a suitable degradation rate for our study, and scaffolds with a mass ratio of 1:1 were therefore chosen for subsequent experiments. The water absorption and porosity ratios of the scaffolds are key features for supporting tissue regeneration. Both these ratios were better for 3D-printed scaffolds than for ordinary scaffolds by freeze-drying technique (Figure 2I and J). We also analyzed the release profiles of exosomes in CC-INExos and 3D-CC-INExos scaffolds. 3D-CC-INExos scaffolds showed sustained exosome release for up to 14 days, and > 80% of the unobstructed loaded exosomes were released (Figure 2K). IF indicated that exosomes released from scaffolds could be phagocytosed by NSCs (Figure 2L).
Figure 3|Good biocompatibility of 3D-CC-INExos scaffolds and effects on neural differentiation of NSCs.
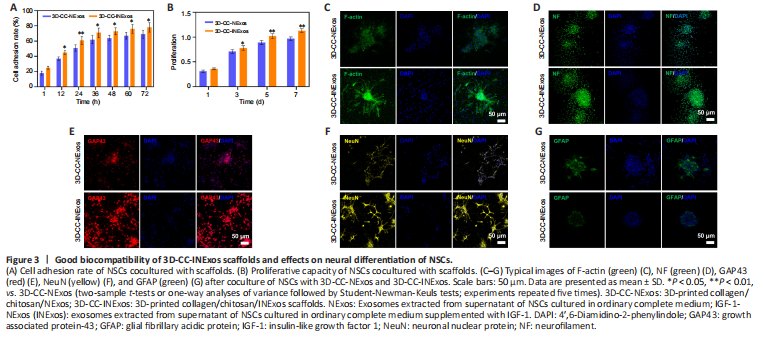
NSCs cocultured with 3D-CC-INExos scaffolds showed better cell adhesion than NSCs cocultured with 3D-CC-NExos scaffolds after coculture for 12 hours (P < 0.05; Figure 3A), and the proliferative capacity of NSCs cocultured with 3D-CC-INExos scaffolds was stronger than that of NSCs cocultured with 3D-CC-INExos scaffolds after 3 days of coculture (P < 0.05; Figure 3B). It is important to maintain the stemness, normal differentiation, and axonal growth of NSCs during the repair process. The fluorescently labelled phalloidin-dye conjugate clearly showed the morphology and distribution of microfilaments in NSCs under CLSM, and the F-actin-positive area was significantly higher in the 3D-CC-INExos compared with the 3D-CC-NExos scaffold group (Figure 3C). Furthermore, the NF-, GAP43-, and NeuN-positive areas were significantly larger in the 3D-CC-INExos compared with the 3D-CC-NExos scaffold group (Figure 3D–F). However, GFAP, as a marker of astrocyte activation, was significantly decreased in the 3D-CC-INExos group compared with the 3D-CC-NExos group (Figure 3G). These results demonstrate that 3D-CC-INExos could facilitate neural differentiation of NSCs while inhibiting astrocyte differentiation compared with 3D-CC-NExos.
Figure 5|Tissue regeneration and reparative ability of scaffolds in vivo 2 months after TBI.
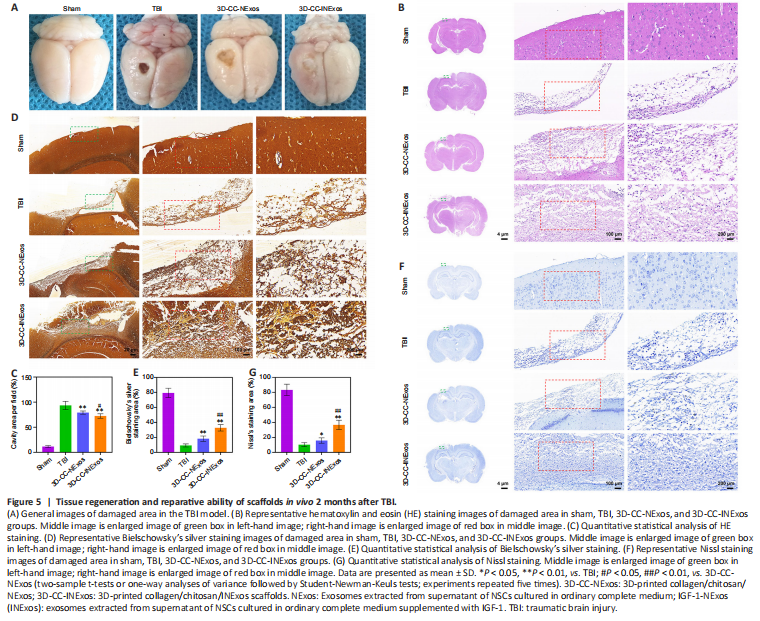
To assess the reparative effects of the scaffolds in the damaged area, we compared the general appearance of the damaged area in the model and the development of new tissue 2 months after TBI by HE, silver, and Nissl staining. There was no loss of brain tissue and no obvious changes in cell morphology in the sham group. However, the damaged tissue was obviously repaired better in the 3D-CC-INExos group compared with the other two experimental groups, based on the general appearance (Figure 5A). Rats in the TBI group showed massive tissue loss evident from the general appearance, and HE staining showed few regenerated cells (Figure 5B and C). In contrast, rats in the 3D-CC-NExos and 3D-CC-INExos groups showed obvious brain tissue regeneration in terms of overall appearance, and HE staining showed regenerated cells in the injured area in the 3D-CC-INExos group. The regenerative effect of 3D-CC-INExos was better than that of 3D-CC-NExos for TBI. The extent of Bielschowsky’s silver staining was significantly wider in the 3D-CC-INExos and 3D-CC-NExos groups than in the TBI group (P < 0.01; Figure 5D and E). These results indicated that the 3D-printed scaffolds improved brain tissue repair. In addition, the number of nerve fibers in the injured area was significantly greater in the 3D-CC-INExos group than in the 3D-CC-NExos group (P < 0.01). Similarly, Nissl bodies, as neuronal markers, were denser and more widely distributed in the injured area in the 3D-CC-INExos group (P < 0.01; Figure 5F and G). These results demonstrated that implantation of the 3D-CC-INExos scaffold had a better effect than the 3D-CC-NExos scaffold on the regeneration of nerve fibers and neurons after TBI.
Figure 6|IF analysis of NSC recruitment in damaged area 2 months post-injury.
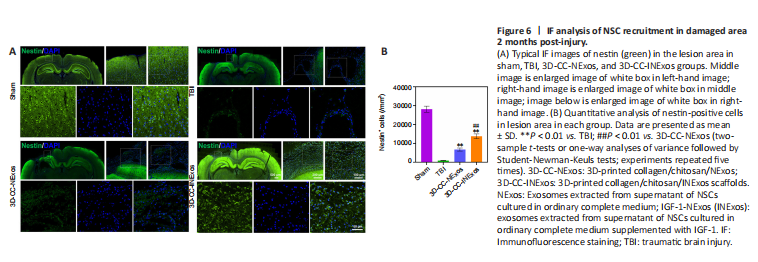
Figure 7|Regeneration of nerve fibers, myelin sheaths, and axons 2 months post-injury.
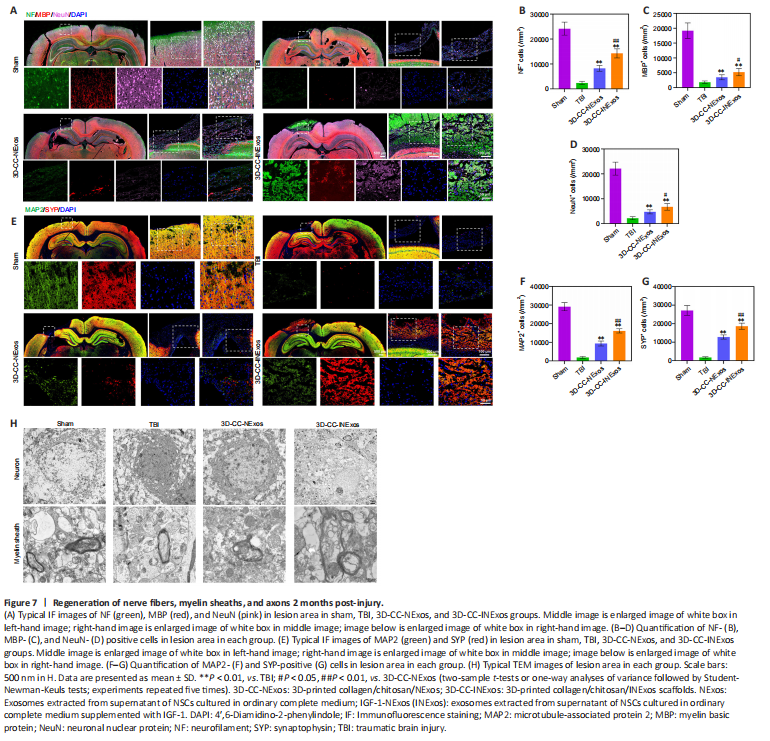
We investigated nerve regeneration of TBI after treatment with 3D-CC-INExos using IF and TEM. IF using an antibody against the NSC marker nestin revealed significantly more NSCs in the damaged area in the 3D-CC-INExos group (P < 0.01; Figure 6A and B), indicating that 3D-CC-INExos scaffolds could recruit NSCs to the injured area after TBI. IF was also carried out using NF, MBP, and NeuN antibodies to assess the regeneration of nerve fibers, myelin sheaths, and mature neurons in the injured area at 2 months post-injury, respectively (Figure 7A). Implantation of the 3D-CC-INExos scaffold in the injured area increased the numbers of nerve fibers, myelin sheaths, and mature neurons compared with the 3D-CC-NExos scaffold (P < 0.05; Figure 7B–D). We further assessed the effect of the scaffolds on synapse formation by dual IF labelling with specific markers (MAP2 and SYP). MAP2- and SYP-positive cells were significantly increased in the 3D-CC-INExos compared with the TBI and 3D-CC-NExos groups (Figure 7E–G). In conclusion, these results demonstrate that implantation of a 3D-CC-INExos scaffold could enhance neural regeneration after TBI. Finally, we observed the neuronal structures, number of axons, and diameter and thickness of the myelin sheath in the injured area 2 months after TBI using TEM (Figure 7H), which showed that implantation of the 3D-CC-INExos scaffold not only ameliorated neuronal structures but also increased the diameter and thickness of the myelin sheath.
Figure 8|Angiogenesis, inflammation, and apoptosis in the injured area 2 months post-injury.

We evaluated the effects of the scaffolds on angiogenesis, inflammation, and apoptosis in the injured area 2 months after TBI by IF and TUNEL staining. More cells expressing CD31 and α-SMA (specific markers of angiogenesis) were found in the injured area of 3D-CC-INExos compared with TBI and 3D-CC-NExos (Figure 8A). These data suggest that implanting 3D-CC-INExos could improve angiogenesis. In addition, expression of CD68 and Iba-1 in the lesion area was decreased in the 3D-CC-INExos group (Figure 8B), indicating that 3D-CC-INExos treatment further suppressed inflammation, and fewer TUNEL-positive signals were captured in the 3D-CC-INExos scaffold group (Figure 8C). These results suggest that 3D-CC-INExos scaffolds could further enhance angiogenesis while suppressing inflammation and inhibiting cell apoptosis in the lesion area.
Figure 9|Biocompatibility of scaffolds in vivo.

Exosomes have demonstrated excellent biocompatibility; however, we further examined the tolerance to the scaffolds in vivo. The scaffolds were implanted into the TBI injury cavity of rats. HE staining showed no pathological abnormalities in the heart, lung, liver, spleen, or kidney in the TBI, 3D-CC-NExos, and 3D-CC-INExos groups compared with the sham group (Figure 9A). Hematological analysis also showed no significant differences in liver and kidney functional indicators in the scaffold groups compared with the sham group (Figure 9B). These results suggested that the scaffolds were biocompatible in vivo.