神经退行性病
-
Figure 1|Typical characteristics of hucMSCs and hucMSC-Exos.
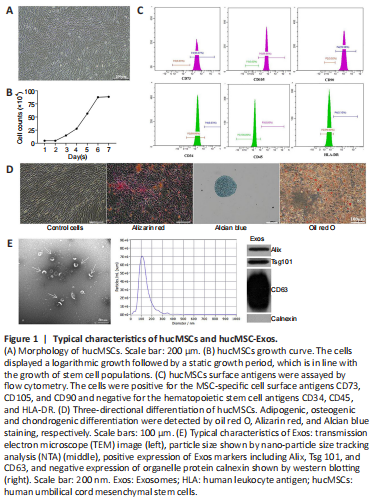
To verify the identity of the isolated mesenchymal stem cells, we observed cell morphology, performed trypan blue staining and cell counts to plot the cell growth curve, and performed flow cytometry analysis of cell surface markers. The cells isolated from the human umbilical cords grew in parallel or spiral shapes (Figure 1A). The cells displayed logarithmic growth followed by a static growth period, which is in line with the growth pattern of stem cell populations (Figure 1B). More than 99.9% of the cells expressed the MSC surface antigens CD73, CD105, and CD90, while less than 0.5% of the cells expressed the hematopoietic stem cell antigens CD34 and CD45 and the human leukocyte antigen (HLA)-DR (Figure 1C). Red calcium deposition was observed during osteogenic induction, a large number of the oil red O–positive lipid droplets were observed during adipogenic induction, and the deposition of acid mucopolysaccharide with positive Alcian blue staining was observed during chondrogenic induction, suggesting multiple differentiation potential (Figure 1D). The above results confirmed that the isolated cells were indeed mesenchymal stem cells.
To identify Exos isolated from hucMSCs, transmission electron microscopy, NTA, and western blot were used to assess cell morphology, particle size, and surface markers, respectively. We found that the extracted Exos had a cup-shaped, cupholder-shaped, or biconcave discoid-shaped double-layer membrane structure (Figure 1E). Overall, 98.1% of the particles were 110.1 nm in size, which is within the normal range for Exos particle size (30–150 nm) (Figure 1E). The Exos were positive for the Exos markers CD63, TSG101, and Alix, and negative for the organelle protein calnexin (Figure 1E). These results confirmed that the Exos were appropriately isolated.
Figure 2|HucMSC-Exos co-localized with DA neurons and microglia in the lesioned substantia nigra improved behavior, and increased the concentrations of DA and its metabolites in lesioned striatum of PD model rats.

An outline of the in vivo experiment is shown in Figure 2A. To investigate whether hucMSC-Exos can cross the BBB to enter the damaged SN, PKH26-labeled Exos were injected via the tail vein or into the lateral ventricle of PD model rats for 24 hours. Immunofluorescence staining showed that hucMSC-Exos colocalized with damaged DA neurons (Figure 2B) and microglia (Figure 2C) in the lesioned SN, suggesting that hucMSC-Exos can be taken up by both cell types. We noticed that the microglia in the control group were unperturbed and extensively ramified, while the microglia in the other three groups exhibited short and bold projections, reflecting microglia activation. The percentage of neuronal loss and microglia activation in the PD model at 3 weeks are shown in Additional Figure 1.
To investigate the effect of hucMSC-Exos on behavior in a 6-OHDA-induced rat model of PD, we assessed APO-induced rotation at 2, 4, 6, and 8 weeks after Exos injection. Compared with the 6-OHDA group, the rotational number in the Exos-LV group decreased significantly from 6 weeks, and that in the Exos-T group decreased significantly from 4 weeks after injection (P < 0.01), and both continued until 8 weeks after injection (P < 0.01). These findings suggest that administration of Exos by both injection methods improved behavior in PD model rats, and there was no significant difference between the two injection methods (Figure 2D).
Next, high performance liquid chromatography-mass spectrometry (HPLC-MS) was used to determine the concentrations of DA, 5-HT, and their metabolites. The molecular structure of all analytes is shown in Figure 2E. 3,4-Dihydroxyphenyl acetic acid (DOPAC) and homovanillic acid (HVA) are metabolites of DA, 5-HT concentration is related to coordinated movement, and 5-HIAA is a metabolite of 5-HT. The HPLC-MS results showed that, compared with the 6-OHDA group, the concentrations of DA, 5-HIAA (P < 0.01), DOPAC, HAV, and 5-HT (P < 0.05) in both injection groups were significantly increased, and there was no significant difference between Exos-T and Exos-LV groups (P > 0.05; Figure 2F).
Figure 3|HucMSC-Exos reduces the damage to DA neuron in the substantia nigra of PD model rats.
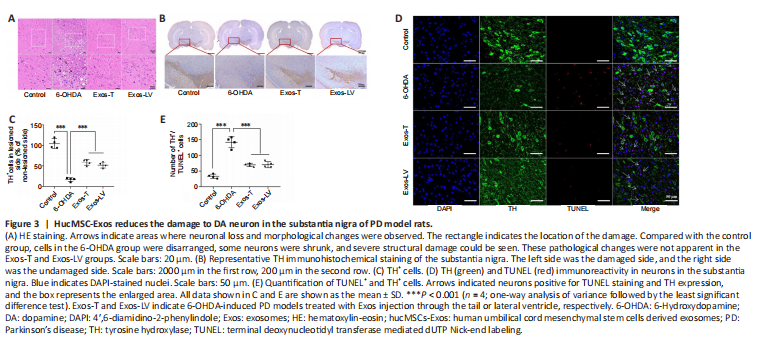
To investigate the protective effect of hucMSC-Exos on DA neurons in the lesioned SN in PD model rats, HE and immunohistochemistry staining were performed. HE staining showed that, in the control group, most neurons were arranged in a regular pattern and contained large, round nuclei. The neuronal architecture was clearly visible and morphologically intact. In the 6-OHDA group, the cells were disarranged. Some neurons were shrunken, the cells stained darkly, and the boundary between the nucleus and cytoplasm was unclear. The interstitial space was relatively loose, and many vacuoles could be observed in the SN. Compared with 6-OHDA-treated rats, the pathological changes were reduced in the Exos-LV and Exos-T groups (Figure 3A). TH immunoreactivity analysis (Figure 3B) showed that 6-OHDA treatment decreased the number of TH neurons in the lesioned SN, while the number of TH neurons in the Exos-LV and Exos-T groups was significantly increased (P < 0.001; Figure 3C).
To further determine the effect of hucMSC-Exos on DA neurons in the PD rat model, TH and TUNEL double immunofluorescence staining was used to assess damage to TH-positive neurons. The results showed that TH and TUNEL double-positive cell numbers were significantly increased in 6-OHDA-treated rats and decreased in Exos-LV and Exos-T rats (both P < 0.001). There was no significant difference in the number of TH and TUNEL double-positive cells between the Exos-LV and Exos-T groups (Figure 3D and E). These data suggest that hucMSC-Exos reduced the damage to DA neurons caused by 6-OHDA.
Figure 4|HucMSC-Exos reduces microglial activation in the substantia nigra of PD model rats.

The effect of hucMSC-Exos on microglial activation in the lesioned SN was determined by TH and Iba-1 double immunofluorescence staining. We found that Iba-1–positive microglia in the control group had long and thin branches, while the 6-OHDA group exhibited a greater number of Iba-1–positive cells, as well as shorter and thicker branches. Compared with the 6-OHDA group, in both the Exos-LV and the Exos-T groups the microglial morphology was more like that seen in the control group, and the microglia had thinner branches (Figure 4A). In addition, the number of microglia was significantly decreased (P < 0.001; Figure 4B). These data suggest that hucMSC-Exos inhibited microglial activation. Rats in the control group exhibited no microglial activation, while in the 6-OHDA group three out of four rats exhibited an intense microglial response, and rats in the Exos-T and Exos-LV groups exhibited mild to moderate microglia responses (Table 1).
Figure 5|HucMSC-Exos are taken up by BV2 cells and mitigate the deleterious effect on SH-SY5Y cell viability caused by growth in conditioned medium from BV2 cells stimulated with LPS.

The morphology of the SH-SY5Y (Figure 5A) and BV2 (Figure 5B) cells and their corresponding cell growth curves are shown. Spent medium from BV2 cells induced with LPS and ATP was added to SH-SY5Y cells, and SH-SY5Y cell viability was assessed by CCK8 assay. We found that conditioned medium from BV2 cells induced with 1 μg/mL LPS and 5 mM ATP for different time periods significantly reduced SH-SY5Y cell viability to 50% at 3 hours, and these conditions were chosen for the in vitro model (Figure 5C).
To determine whether Exos have a protective effect on injured neurons in vitro, Exos uptake by BV2 cells was first observed by confocal microscopy. Exos were taken up in small amounts by normal BV2 cells at 1 hour and in large amounts at 3 hours and later (Figure 5D). Exos were also taken up by BV2 after drug intervention at 24 hours, and bright field microscopy showed that the Exos entered the interior of the cells (Figure 5D). Furthermore, confocal microscopy with 2.5D and 3D scanning confirmed that the Exos entered the nucleus at 3 hours (Figure 5D).
To assess the protective effect of Exos on the neuroinflammatory cell model, we first established a time-concentration curve for the activity on Exos on normal BV2 cells. Exos at 0–100 μg/mL did not affect BV2 cell viability for 48 hours (Figure 5E), which suggested that the Exos had no toxicity for normal BV2 cell. Conditioned medium from BV2 cells pretreated with Exos at 50–100 μg/mL and stimulated with LPS and ATP significantly increased SH-SY5Y viability compared with condition medium from BV2 cells that were stimulated but not pretreated (P < 0.05). There was no significant difference in the results between 75 and 100 μg/mL Exos (P > 0.05; Figure 5F). Thus, 75 μg/mL Exos was selected for use in subsequent experiments. These data suggest that Exos protect neurons by inhibiting activation of LPS+ATP-induced microglia.
Figure 6|HucMSC-Exos decreases IL-1β and IL-18 secretion by BV2 cells and prevents the adoption of pyroptosis-associated morphology by BV2 cells.
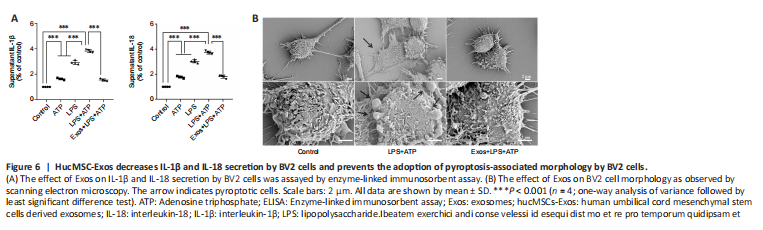
To further investigate the effect of Exos on a BV2 cell model of neuroinflammation, ELISA and scanning electron microscopy were used. The ELISA results showed that IL-1β and IL-18 secretion increased significantly when BV2 cells were stimulated with LPS and ATP, and pretreatment with Exos significantly inhibited this effect (both P < 0.001; Figure 6A).
Cell swelling and the loss of cell membrane integrity are the main characteristics of pyroptosis. The scanning electron microscopy results showed that cell morphology in the control group was normal, and the cell contours were clear. In the LPS + ATP group, the cell morphology was irregular, swollen, and enlarged, and cells were attached to the climbing films. Vesicles generated by cytoplasmic overflow were observed, which indicated that membrane integrity was disrupted. Pretreatment with Exos decreased the number of vesicles and prevented the observed changes in cell morphology (Figure 6B).