脑损伤
-
Figure 2|Vav1 knockdown decreases brain infarction area and neuronal injury in MCAO/R rats.
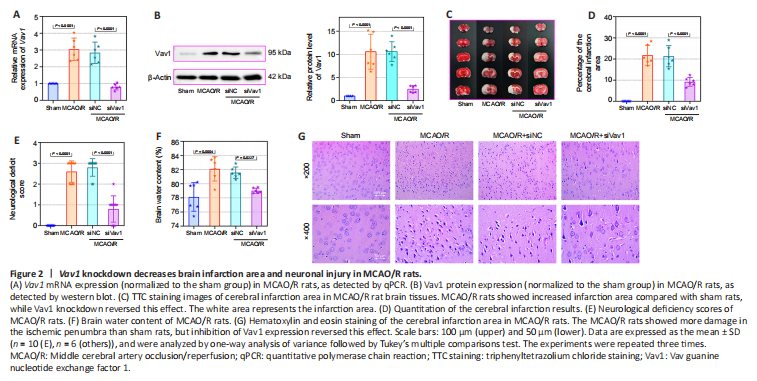
Next, we inhibited Vav1 expression in MCAO/R rats to explore the effect of Vav1 on brain infarction and neuronal injury after cerebral I/R. While Vav1 mRNA (Figure 2A) and protein (Figure 2B) expression levels were significantly increased in MCAO/R rats compared with sham rats, this effect was reversed by inhibition of Vav1. Then, we examined the cerebral infarction area in rat brain tissues by TTC staining. The images (Figure 2C) and quantitative results (Figure 2D) clearly showed that the infarction area in the MCAO/R rats was larger than that in sham rats, and this difference was strikingly decreased by Vav1 knockdown. In addition, inhibiting Vav1 expression ameliorated the neurological deficits seen in MCAO/R rats, as determined by neurological deficiency scoring (Figure 2E). When we assessed the brain water content, we found that it was higher in MCAO/R rats than in sham rats, and that inhibition of Vav1 dramatically reversed this effect (Figure 2F). Finally, hematoxylin and eosin staining was used to analyze tissue damage in the ischemic penumbra of MCAO/R rats. Compared with the sham group, rats in the MCAO/R group showed aggravated damage in the ischemic penumbra, but inhibition of Vav1 expression decreased this damage (Figure 2G). Taken together, these findings indicate that Vav1 knockdown decreases brain infarction and neuronal injury in MCAO/R rats.
Figure 3|Vav1 knockdown alleviates neuronal loss and apoptosis in the ischemic penumbra of MCAO/R rats.
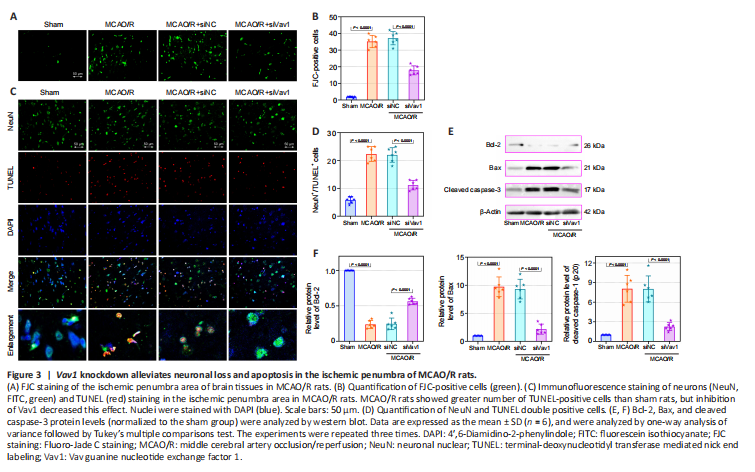
Cerebral I/R injury often results in neuronal degeneration and death (Xu et al., 2007; Liang et al., 2008), and therefore we used FJC and immunofluorescence staining to explore the role of Vav1 in neuronal loss and apoptosis. Very little FJC staining was seen in sham rats, while FJC-positive neurons were clearly visible in MCAO/R rats (Figure 3A and B). However, Vav1 inhibition clearly decreased the number of FJC-positive cells in MCAO/R rats compared with the negative control.
Next, we explored the effect of Vav1 on neuronal apoptosis. Immunofluorescence staining for NeuN, a neuronal marker (Lavezzi et al., 2013), and TUNEL staining were used to detect neuronal apoptosis in the ischemic penumbra. MCAO/R resulted in a clear increase in the number of TUNEL-positive cells compared with sham rats (Figure 3C and D). However, Vav1 knockdown decreased the number of TUNEL-positive cells in MCAO/R rats compared with the negative control. We also measured the expression of several intrinsic apoptotic markers, including Bcl-2, Bax, and cleaved caspase-3 (Figure 3E and F). Inhibition of Vav1 significantly reversed the changes in the expression level of Bcl-2, Bax and cleavage of caspase-3, which was induced by MCAO/R. These results suggested that inhibiting Vav1 expression decreases neuronal apoptosis in the brains of MCAO/R rats.
Figure 4|Vav1 knockdown represses the activation of microglia and NLRP3 inflammasome in the ischemic penumbra of MCAO/R rats.

Inflammation after cerebral ischemia is an important factor leading to cerebral I/R injury (Li et al., 2021). As the resident macrophages of the central nervous system, microglia are involved in the inflammatory response to I/R (Wang et al., 2022). To determine whether microglia and the inflammasome were activated by MCAO/R in our rat model, we assessed expression of the microglial markers Iba-1 and Vav1, as well as NLRP3, an integral component of the inflammasome. The numbers of Iba-1/Vav1 double positive cells were increased in MCAO/R rats compared with sham rats, and inhibiting Vav1 decreased this effect (Figure 4A and B). Merged images showed that Iba-1 and Vav1 co-localized more extensively in MCAO/R rats than in sham rats. The number of NLRP3-positive cells was higher in MCAO/R rats than in sham rats, while Vav1 inhibition reversed this effect (Figure 4C and D). Furthermore, as seen in Figure 4C, there was substantial co-localization of Iba-1 and NLRP3 in MCAO/R rats.
Figure 6|Increased Vav1 expression in BV-2 cells subjected to OGD/R.
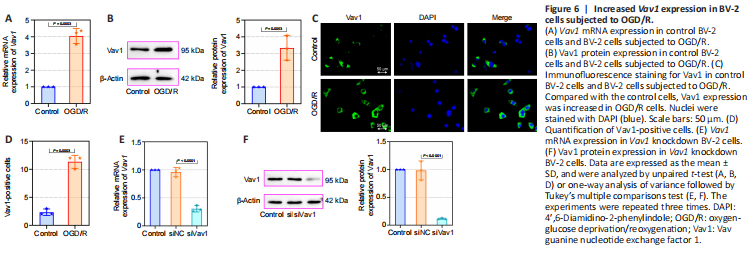
Next, we constructed an OGD/R cell model in mouse BV-2 microglia cells to evaluate the effects of Vav1 in vitro. First, we assessed Vav1 mRNA and protein levels in the cells. Consistent with the results from the in vivo experiments, Vav1 expression was increased in BV-2 cells after OGD/R compared with the control cells (Figure 6A and B). Immunofluorescence staining confirmed that there were more Vav1-positive cells in the OGD/R group than in the control group (Figure 6C and D). Then, Vav1 was silenced for further investigation (Figure 6E and F).
Figure 7|Vav1 knockdown represses inflammation in BV-2 cells subjected to OGD/R.
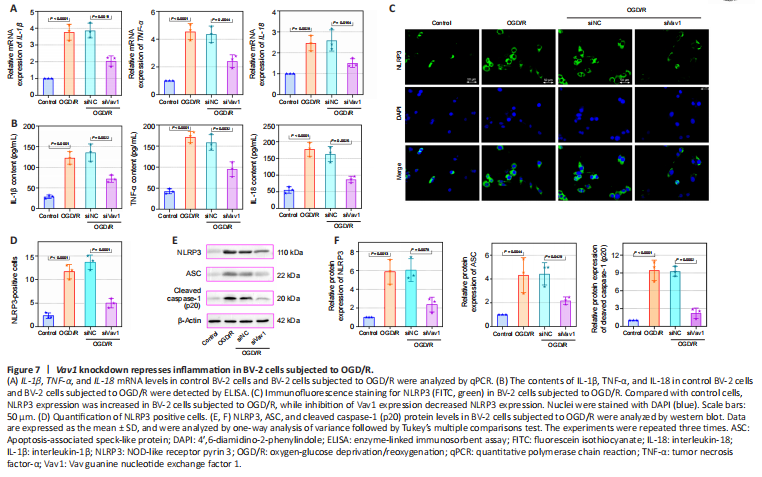
The mRNA levels and contents of the IL-1β, TNF-α, and IL-18 were also detected in vitro. Compared with the control, IL-1β, TNF-α, and IL-18 mRNA and contents were increased in BV-2 cells subjected to OGD/R, while this increase was alleviated by the inhibition of Vav1 (Figure 7A and B). Furthermore, more NLRP3-positive cells were seen in the OGD/R cells compared with the control cells (Figure 7C and D), while Vav1 inhibition reversed this effect. Similar to the in vivo results, silencing Vav1 reversed the changes in NLRP3, ASC, and cleaved caspase-1 (p20) levels observed in BV-2 cells subjected to OGD/R (Figure 7E and F). These findings confirm that Vav1 knockdown represses inflammation in vitro.