脑损伤
-
Figure 4|Sirt6 expression in the PrL after chronic sleep deprivation treatment via immunohistochemistry (A and B) and western blot assay (C and D).
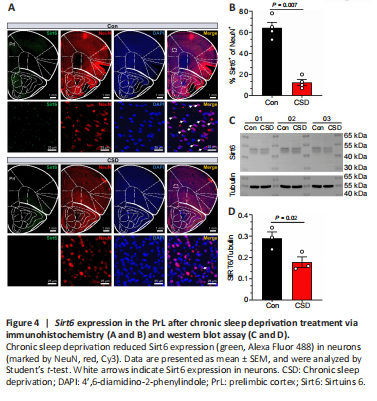
A previous study demonstrated that CSD could lead to a relative decrease in cerebral glucose metabolism (Wu et al., 1991). Sirt6 is important for glucose homeostasis in the liver (Kim et al., 2010), and is highly expressed in the immature brain (Garcia-Venzor and Toiber, 2021). Thus, we measured Sirt6 protein expression in the PrL to determine the influence of CSD. As shown in Figure 4A, the Sirt6 protein was primarily restricted to the cell nuclei. Compared with the animals in the control group, the CSD mice exhibited significantly reduced (P < 0.05) Sirt6 expression in the PrL, as demonstrated by immunohistochemistry (Figure 4A and B) and western blotting (Figure 4C and D).
Figure 5|Sirt6 overexpression in PrL neurons.
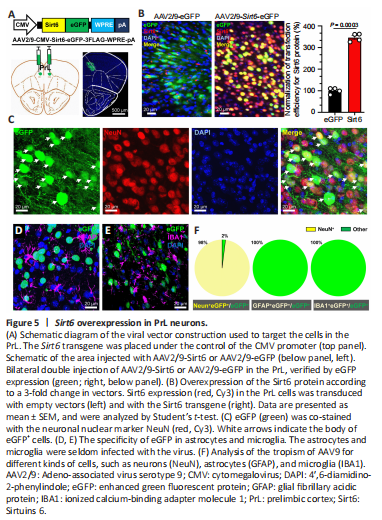
To generate the Sirt6 overexpression animal model, we delivered adeno-associated virus serotype 9 (AAV2/9) encoding Sirt6-eGFP fusion protein under the control of the CMV promoter to the PrL (Figure 5A). We sacrificed the mice for brain collection 21 days after the virus infection, and assessed virus overexpression and cell tropism. As expected, exogenous Sirt6 was dramatically expressed and restricted in the nuclei (Figure 5B). The normalized transfection efficiency of Sirt6 was significantly increased to 3.46 ± 0.11 in the Sirt6-eGFP group compared with the fluorescence intensity (1.00 ± 0.08) in the control eGFP group (P < 0.01). Within the virally transduced region, the Sirt6 overexpression was mainly observed in neurons (Figure 5C) with high specificity (Figure 5D–F). Co-staining with the microglial marker IBA1 (Zhang and Cui, 2021) or the astrocytic marker GFAP (Yu et al., 2020) showed that AAV9 infrequently underwent glial transduction (Figure 5D–F).
Figure 9|Sirt6 overexpression ameliorates synaptic dysfunction in chronic sleep deprived mice.
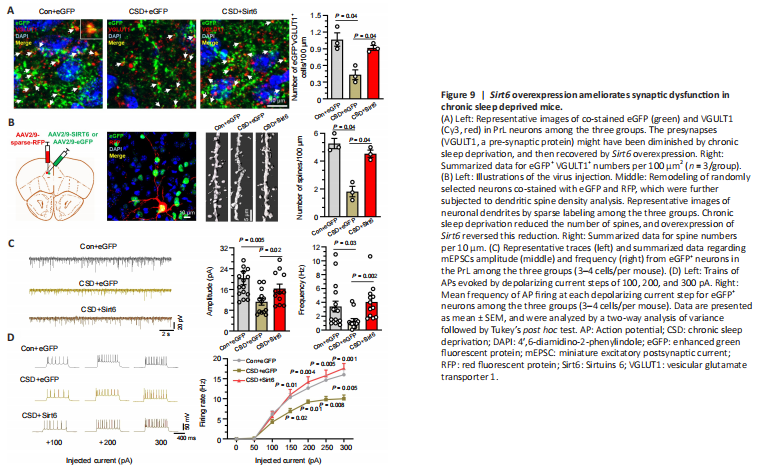
To test our hypothesis, we stained sections containing PrL regions with VGLUT1 antibody. As shown in Figure 9A, VGLUT1 appeared as red fluorescent puncta in brain sections (Figure 9A), and the puncta co-labeled with eGFP were considered to be VGULT1 from AAV-infected neurons. Compared with the control group, CSD significantly reduced (P < 0.05) the number of VGLUT1-positive puncta overall and that in AAV infected neurons. This reduction was diminished in neurons overexpressing Sirt6, as indicated by a significant increase in eGFP-VGLUT1 double-positive puncta in the CSD + Sirt6 group compared with the CSD + eGFP group (P < 0.05; Figure 9A).
Next, we examined the dendritic spine densities in the eGFP-expressing neurons using the sparse-labeling method. The results showed that the CSD mice had a significantly decreased number of dendritic spines compared with the control group (P < 0.05). Meanwhile, mice overexpressing Sirt6 had significantly higher spine densities than CSD mice (P < 0.05; Figure 9B).
In the patch-clamp experiment, eGFP+ neurons in CSD mice showed a significant reduction in the frequency and amplitude of mEPSCs (P < 0.05) compared with those in the control group. Sirt6 overexpression was sufficient to prevent falls in the frequency and amplitude of mEPSCs after CSD (P < 0.05, Figure 9C). Furthermore, we recorded trains of action potentials evoked by applying depolarizing currents at 50 mV increments to assess the firing rate of the eGFP+ neurons in the three groups. Compared with the control animals, the total numbers of action potentials generated by the stimulation currents (150, 200, 250, and 300 pA) were significantly lower (P < 0.05) in the neurons from CSD mice, as shown in the f-I plot in Figure 9D. Consistently, Sirt6 overexpression could reverse the CSD-induced decrease in action potential firing rates in eGFP+ neurons (Figure 9D).