脑损伤
-
Figure 2|MS-hippocampus cholinergic transmission is degraded in chronic epileptic mice.
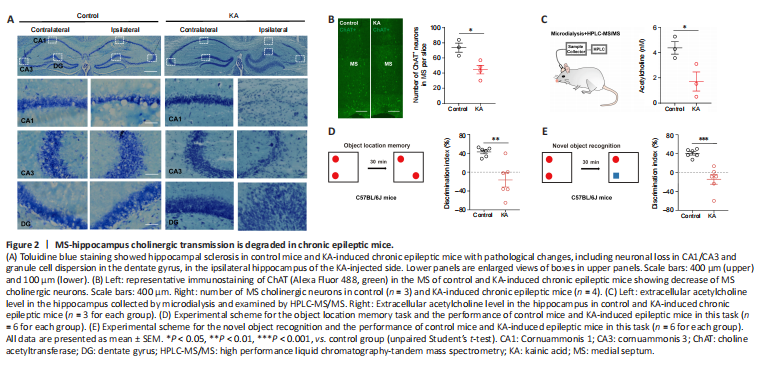
To verify the pathological changes of MS-hippocampus tracts in an animal model of epilepsy, we used an intra-hippocampal KA-induced epileptic mouse model, which has been reported to resemble the typical patterns of hippocampal pathology (Lévesque and Avoli, 2013) and impaired cognitive function (Wang et al., 2021). In our study, toluidine blue staining verified the pathological changes, including neuronal loss in CA1/CA3 and granule cell dispersion in the dentate gyrus, in the ipsilateral hippocampus of the KA-injected side in KA-induced chronic epileptic mice, compared with the contralateral hippocampus and that in control mice (Figure 2A).
The hippocampus, which receives cholinergic projection from the MS, is not only generally recognized as a region for encoding learning and memory, but also as one of the most common epileptogenic focuses in TLE (Bui et al., 2018). We speculated that the MS-hippocampus is a key node for the generation of cognitive impairments in TLE. We first detected the expression of MS cholinergic neurons in TLE. Immunohistochemical staining showed that there was a significant decrease in the number of MS cholinergic neurons (ChAT-positive cells) in KA-induced epileptic mice compared with control mice (Figure 2B, P = 0.0176). Because ACh in the hippocampus is mainly released from MS-hippocampus cholinergic projections, we measured the extracellular ACh level in the ipsilateral hippocampus of the KA-injected side using a microdialysis component in combination with HPLC-MS/MS. The extracellular ACh level in the hippocampus was significantly lower in KA-induced chronic epileptic mice than in control mice (Figure 2C, P = 0.0418), which suggested decreased hippocampal ACh release in TLE model mice. Next, we evaluated cognitive function in control mice and KA-induced epileptic mice by using the OLM and NOR memory tasks. KA-induced epileptic mice showed cognitive impairments with low discrimination index in both OLM and NOR (Figure 2D, P = 0.0034; Figure 2E, P = 0.0006). The above results indicate pathological changes of MS cholinergic neurons and cognitive impairments in the TLE model that were in accordance with the above clinical findings.
Figure 3|Ablation of MS cholinergic neurons impairs cognitive function.
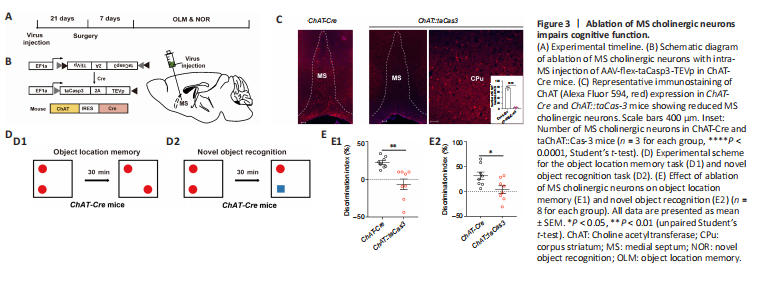
To further confirm the causal role of MS cholinergic neuronal loss in TLE-associated cognitive impairments (Figure 3A), a Cre-dependent adeno-associated virus (AAV-flex-taCasp3-TEVp) was microinjected into the MS of ChAT-Cre mice (referred to as ChAT::taCasp3 mice; Figure 3B) to selectively ablate MS cholinergic neurons. Immunohistochemical staining showed that 93% of MS cholinergic neurons were ablated in ChAT::taCasp3 mice, whereas those in corpus striatum, a nearby region that also is enriched in cholinergic neurons, were not affected (Figure 3C, ChAT-Cre group vs. ChAT::taCas3 group, P < 0.0001). Then, we examined learning and memory processes in ChAT-Cre mice and ChAT::taCasp3 mice using OLM and NOR memory tasks, respectively (Figure 3D). The results showed that ChAT::taCasp3 mice with ablated MS cholinergic neurons had low discrimination index in both OLM and NOR, similar to that of epileptic mice (Figure 3E1, ChAT-Cre group vs. ChAT::taCas3 group, P = 0.0018; Figure 3E2, ChAT-Cre group, ChAT::taCas3 group, P = 0.0210). The above results showed that MS cholinergic neuronal loss alone was sufficient to impair cognitive function, which mimicked the impairments of KA-induced epileptic mice. These results suggested that MS cholinergic neuron loss in epilepsy might mediate the generation of cognitive impairments in TLE.