周围神经损伤
-
Figure 1|Analysis of mRNA expression profiles detected by transcriptome sequencing following spinal cord injury.
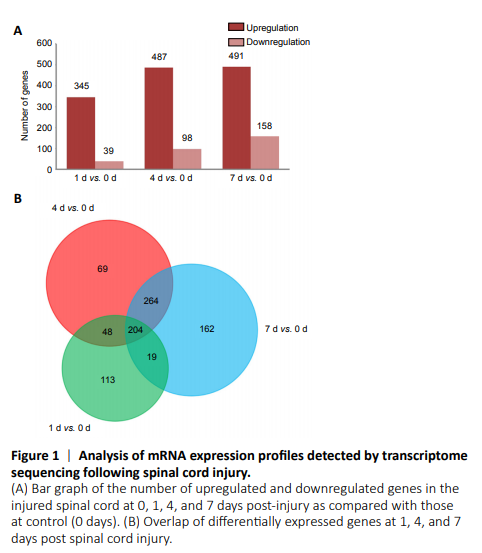
Figure 2|Heatmap and cluster dendrogram of integrated differentially expressed genes involved in inflammatory responses at 0, 1, 4, and 7 days following spinal cord injury.
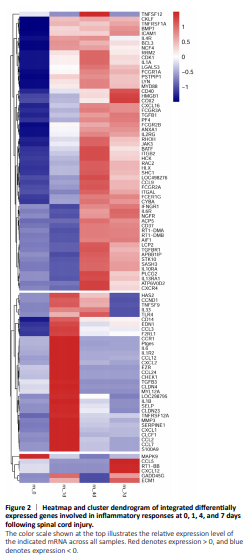
Figure 3|A reconstructed gene network was created using the Ingenuity Pathway Analysis software on the basis of integrated differentially expressed genes involved in inflammatory responses.
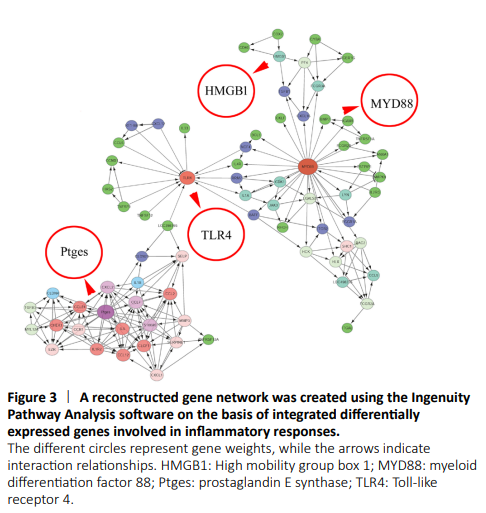
To reveal the potential regulatory mechanism of HMGB1 in the inflammatory response of astrocytes, we analyzed the gene expression profiles of the rat spinal cord following injury at 0, 1, 4, and 7 days by transcriptome sequencing. A total of 1618 DEGs with a greater or less than twofold change were identified (Figure 1A and Additional Table 1). GO analysis revealed that these DEGs were significantly enriched in T cell immune response, immune cell differentiation, prostaglandin secretion, inflammatory cell aggregation, and chemokine activation (Additional Figure 1). KEGG analysis identified that signaling pathways relevant to TLRs, cytokine-cytokine receptor interaction, and arachidonic acid metabolism were included in the top 30 significant functional enrichment results (Additional Figure 1). We further integrated these DEGs at different time points to narrow the scope of bioinformatic analysis and characterized 204 functional genes, among which 97 DEGs were involved in the inflammatory response and cell chemotaxis (Figure 1B). These 97 DEGs displayed dynamic alteration following SCI, as presented by the heat map (Figure 2). HMGB1 is known as a key pro-inflammatory factor that evokes an inflammatory response through binding with TLR2/4 or RAGE (Park et al., 2004; Paudel et al., 2019b). To understand HMGB1-mediated intracellular signaling in astrocytes, we performed IPA based on inflammation-related DEGs integrated at 1, 4, and 7 days following SCI. A reconstructed gene network was created, identifying that COXs (also known as Ptges), TLR4, and myeloid differentiation factor 88 were exclusively highlighted as the prominent amplifiers of inflammatory signaling with a core regulator for HMGB1 (Figure 3). The transcriptome profile analysis of SCI indicated that the pro-inflammatory factor HMGB1 may be involved in the activation of COX enzymes via TLR4.
Figure 4|Protein expression changes in HMGB1, TLR4, COXs, and PGESs in the injured spinal cord of rats following spinal cord injury.

To validate the involvement of the HMGB1/TLR4 axis in the regulation of COX expression in the astrocytes, we first detected the temporal changes in HMGB1, TLR4, COX1, and COX2, as well as the isoforms of PGE2 synthase in the injured spinal cord segments at 0, 1, 4, and 7 days following SCI. Western blot analysis revealed that HMGB1 and TLR4 expression levels were significantly upregulated and peaked at 4 and 7 days, respectively (Figure 4A–C). Meanwhile, COX2 and mPGES-1, but not COX1, mPGES-2, or cPGES, were inducibly expressed following SCI with a peak level at 1 day (Figure 4D–H).
Figure 5|Co-localization of TLR4 and COX2 with astrocytes in the injured spinal cord of rats following SCI.
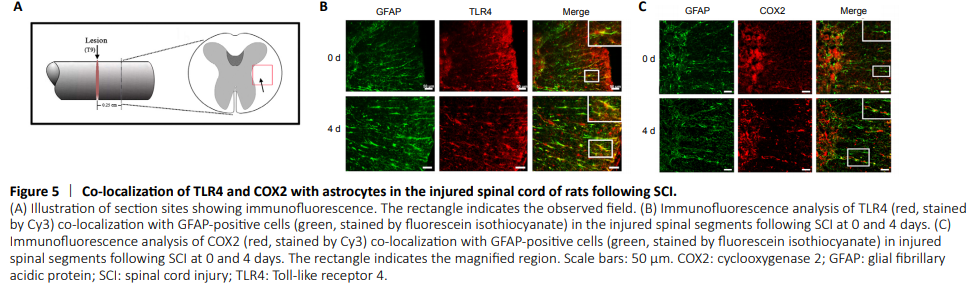
Subsequently, immunofluorescence staining was used to clarify that COX2 upregulation was astrocyte-related and a response to HMGB1 stimulation. As shown in Figure 5, both TLR4 and COX2 colocalized with GFAP-positive cells in the injured spinal cord, indicating a potential correlation of the HMGB1/TLR4 axis and COX2 expression in astrocytes.
Figure 6|Effects of rHMGB1 on the expression of PGE2 synthesis-related proteins in astrocytes.

Next, we sought to confirm the regulatory relationship between HMGB1 and COX2 in astrocytes. Primary cultured astrocytes with a purity of over 90% were stimulated with 0, 1, 10, 100, 1000, or 2000 ng/mL of rHMGB1 (Figure 6A). After exposure to rHMGB1 for 24 hours, the protein levels of COX1, COX2, and the isoforms of PGE2 synthase were detected by western blots. As shown in Figure 6B–G, rHMGB1 significantly induced COX2 and mPGES-1 expression without affecting COX1, mPGES-2, or cPGES expression, which was consistent with the in vivo results.
Figure 7|Effects of TLR2/4 inhibitors on HMGB1-induced COX2 activation and PGE2 production in astrocytes.

To ascertain whether HMGB1-mediated COX2 elevation is TLR-dependent, astrocytes were treated with 10 nM atractylenolide I or 10 nM C29 for 24 hours in the presence of 500 ng/mL of rHMGB1. The COX2 protein level was determined, and the results showed that the addition of atractylenolide I or C29 efficiently attenuated rHMGB1-induced activation of COX2 (Figure 7A and B). An ELISA was subsequently performed to detect PGE2 production in astrocytes. As shown in Figure 7C and D, rHMGB1 could facilitate PGE2 production in astrocytes, and application of 10 nM atractylenolide I or 10 nM C29 accordingly abrogated the stimulatory effects of rHMGB1. The results indicate that HMGB1 activates the COX2/mPGES1/PGE2 cascade in astrocytes via TLR2/4.